Novel frontiers through nitrogen substitution at 6th, 10th and 11th position of artemisinin: Synthetic approaches and antimalarial activity
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Malaria pertains to an array of catastrophic illnesses spurred on by the Plasmodium spp. Artemisinin (ART) is currently prescribed in conjunction with another medication as part of therapeutic regimens for acute malaria. These currently prescribed pharmaceuticals have been around for a while, even after lack of required thermos-metabolic stabilities, alongside fresh proclaims about surfacing resistance and neurotoxicity linked with sequential administration of such combination therapies. Over the years, ARTs seem to have gained popularity through the accelerated reduction in parasitaemia, thus dictating use of differentially stable ART derivatives, in combination or alone, to control the proliferation of malaria. The endoperoxide bridge in the ART pharmacophore plays a non-negotiable role in its action against multiple stages in the parasitic life cycle. However, shorter half-lives and limited bioavailability tend to open doors for another class of endoperoxides. Nitrogen substitution at 6th, 10th and 11th positions of ART draws attention as the best replacements through their disparate stabilities and inability to demonstrate in vivo hydrolytic decomposition into DHA. Discussions pertaining such azaartemisinins and aminoartemisinins reported over the past 30 years have been strongly focused upon, on account of their synthetic methodologies and antimalarial efficacies, in order to assign future candidature to the meritorious moiety.
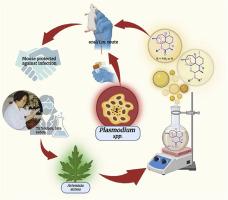
青蒿素第 6、10 和 11 位氮取代的新前沿:合成方法和抗疟活性
疟疾是由疟原虫引起的一系列灾难性疾病。 目前,青蒿素(ART)与另一种药物联合使用,作为急性疟疾治疗方案的一部分。即使在缺乏所需的热代谢稳定性之后,这些目前的处方药已经存在了一段时间,同时还出现了关于抗药性和与连续服用此类联合疗法相关的神经毒性的新说法。多年来,抗逆转录病毒疗法似乎因能加速减少寄生虫血症而广受欢迎,因此需要使用具有不同稳定性的抗逆转录病毒疗法衍生物,通过联合或单独使用来控制疟疾的扩散。抗逆转录病毒疗法药理结构中的内过氧化物桥在对寄生虫生命周期的多个阶段起着不可忽视的作用。然而,较短的半衰期和有限的生物利用度往往为另一类内过氧化物打开了大门。ART 第 6、第 10 和第 11 位的氮替代物因其不同的稳定性和无法在体内水解分解为 DHA 而成为最佳替代物。过去 30 年中报道的此类杂青蒿素类和氨基青蒿素类药物,由于其合成方法和抗疟药效,引起了人们的强烈关注,目的是为未来的候选药物确定有价值的分子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


