Development of selective sigma-1 receptor ligands with antiallodynic activity: A focus on piperidine and piperazine scaffolds
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The design and synthesis of a series of piperidine and piperazine-based derivatives as selective sigma receptor (SR) ligands associated with analgesic activity, are the focus of this work. In this study, affinities at S1R and S2R were measured, and molecular modeling studies were performed to investigate the binding pose features. The most promising compounds were subjected to in vitro toxicity testing and subsequently screened for in vivo analgesic properties. Compounds 12a (AD353) and 12c (AD408) exhibited negligible in vitro cellular toxicity and high potency both in a model of capsaicin-induced allodynia and in PGE2-induced mechanical hyperalgesia. Functional activity experiments showed that S1R antagonism is needed for the effects of these compounds, since the effect was reversed by PRE-084 or absent in KO mice. In addition, 12a exhibited a favorable pharmacokinetic profile, confirming its therapeutic value in treating allodynic conditions. Moreover, a computational model was developed in order to help the understanding about the mechanism of action of most active compounds.
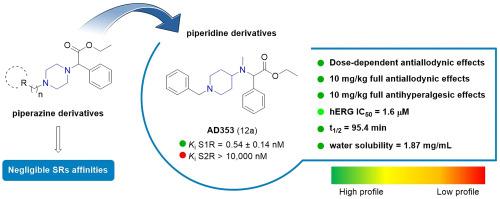
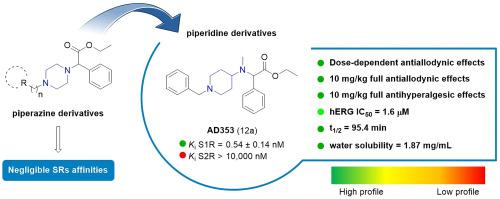
开发具有抗镇痛活性的选择性 sigma-1 受体配体:聚焦哌啶和哌嗪支架
本研究的重点是设计和合成一系列哌啶和哌嗪基衍生物,作为具有镇痛活性的选择性σ受体(SR)配体。在这项研究中,测量了 S1R 和 S2R 的亲和力,并进行了分子建模研究,以探究其结合姿态特征。最有希望的化合物接受了体外毒性测试,随后进行了体内镇痛特性筛选。化合物 12a(AD353)和 12c(AD408)的体外细胞毒性可忽略不计,在辣椒素诱导的异痛症模型和 PGE2 诱导的机械痛觉减退模型中均表现出很高的效力。功能活性实验表明,这些化合物的作用需要 S1R 拮抗,因为 PRE-084 会逆转这种作用,或者 KO 小鼠体内不存在这种作用。此外,12a 表现出良好的药代动力学特征,证实了其在治疗异动症方面的治疗价值。此外,还建立了一个计算模型,以帮助理解大多数活性化合物的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


