Multi-Modal Analysis of human Hepatic Stellate Cells identifies novel therapeutic targets for Metabolic Dysfunction-Associated Steatotic Liver Disease
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background and aims
Metabolic dysfunction-associated steatotic liver disease (MASLD) ranges from Metabolic dysfunction-associated steatotic liver (MASL) to Metabolic dysfunction-associated steatohepatitis (MASH) with fibrosis. Activation of Hepatic Stellate Cells (HSCs) into fibrogenic myofibroblasts plays a critical role in the pathogenesis of MASH liver fibrosis. We compared transcriptome and chromatin accessibility of human HSCs from NORMAL, MASL, and MASH livers at single cell resolution. We aimed to identify genes that are upregulated in activated HSCs and to determine which of these genes are key in the pathogenesis of MASH fibrosis.Methods
18 human livers were profiled using single-nucleus (sn)RNA-seq and snATAC-seq. High priority targets were identified, then tested in 2D human HSC cultures, 3D human liver spheroids, and HSC-specific gene knockout mice.Results
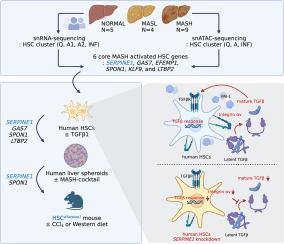
MASH-enriched activated (A) HSC subclusters are the major source of extracellular matrix proteins. We identified a set of concurrently upregulated and more accessible core genes (GAS7, SPON1, SERPINE1, LTBP2, KLF9, EFEMP1) that drive activation of (A) HSC subclusters. Expression of these genes was regulated via crosstalk between lineage-specific (JUNB/AP1), cluster-specific (RUNX1/2) and signal-specific (FOXA1/2) transcription factors. The pathological relevance of the selected targets, such as SERPINE1 (PAI-1), was demonstrated using dsiRNA-based HSC-specific gene knockdown or pharmacological inhibition of PAI-1 in 3D human MASH liver spheroids, and HSC-specific Serpine1 knockout mice.Conclusion
This study identified novel gene targets and regulatory mechanisms underlying activation of MASH fibrogenic HSCs and demonstrated that genetic or pharmacological inhibition of select genes suppressed liver fibrosis.Impact and implications
Here we present snRNA-seq and snATAC-seq analysis of human HSCs from NORMAL, MASL, and MASH livers. We identified additional subclusters that were not detected by previous studies and characterized the mechanism by which HSCs activate in the MASH livers, including the transcriptional machinery that activates HSCs into myofibroblasts. For the first time, we described the pathogenic role of activated HSC-derived PAI-1 (a product of SERPINE1 gene) in the development of MASH liver fibrosis. Targeting of RUNX1/2-SERPINE1 axis may provide a novel strategy for treatment of liver fibrosis in patients.
人类肝星状细胞的多模式分析为代谢功能障碍相关性脂肪肝确定了新的治疗靶点
背景和目的代谢功能障碍相关性脂肪性肝病(MASLD)包括从代谢功能障碍相关性脂肪肝(MASL)到伴有纤维化的代谢功能障碍相关性脂肪性肝炎(MASH)。肝星状细胞(HSCs)活化成纤维化肌成纤维细胞在MASH肝纤维化的发病机制中起着关键作用。我们以单细胞分辨率比较了来自正常肝脏、MASL肝脏和MASH肝脏的人类造血干细胞的转录组和染色质可及性。我们的目的是找出活化的造血干细胞中上调的基因,并确定这些基因中哪些是MASH肝纤维化发病机制中的关键基因。结果MASH富集的活化(A)造血干细胞亚簇是细胞外基质蛋白的主要来源。我们发现了一组同时上调且更容易获得的核心基因(GAS7、SPON1、SERPINE1、LTBP2、KLF9、EFEMP1),它们驱动着(A)造血干细胞亚簇的活化。这些基因的表达是通过细胞系特异性转录因子(JUNB/AP1)、细胞集群特异性转录因子(RUNX1/2)和信号特异性转录因子(FOXA1/2)之间的相互作用来调节的。利用基于dsiRNA的造血干细胞特异性基因敲除或药物抑制PAI-1,在三维人MASH肝球和造血干细胞特异性Serpine1基因敲除小鼠中证实了所选靶点(如SERPINE1(PAI-1))的病理相关性。影响和意义在此,我们对来自NORMAL、MASL和MASH肝脏的人类造血干细胞进行了snRNA-seq和snATAC-seq分析。我们发现了以往研究未检测到的其他亚群,并描述了造血干细胞在 MASH 肝脏中的活化机制,包括将造血干细胞活化为肌成纤维细胞的转录机制。我们首次描述了活化的造血干细胞衍生的 PAI-1(SERPINE1 基因的产物)在 MASH 肝纤维化发展过程中的致病作用。以RUNX1/2-SERPINE1轴为靶点可能为治疗患者肝纤维化提供一种新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


