Thermodynamically Controlled and Industrially Viable Telescopic Process for the Synthesis of Fluazuron
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
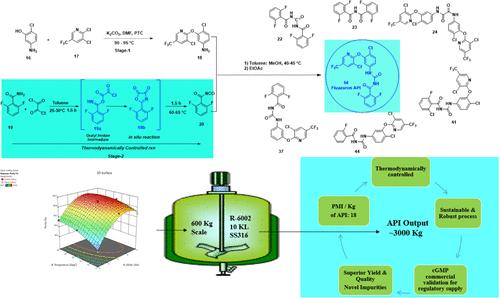
Fluazuron N-[(4-chloro-3-[3-chloro-5-(trifluoromethyl)pyridine-2-yl]oxy phenyl]carbamoyl]-2,6-difluorobenzamide (14) is a noteworthy antiparasitic veterinary medicine belonging to the class of benzoyl phenyl urea derivatives, a class of chitin synthesis inhibitors. The commercial-scale synthesis, which is compliant with current regulatory requirements, particularly purity and impurity profiles, is not well established. Therefore, a robust and sustainable manufacturing process is essential to manufacture and supply fluazuron or any drug substance, for that matter, meeting all criteria. In this work, a safe, scalable, economic, and sustainable process was described through a robust in situ protocol for the bottleneck isocyanate intermediate (20) to manufacture a substantially pure fluazuron active pharmaceutical ingredient (API) with >99.5% HPLC purity and a yield of >78% overall. This large-scale GMP manufacturing process was established by implementing DoE tools and principles of green chemistry like process mass intensity assessment (PMI) and the “3Rs” principle (reduce/reuse/recycle) to attain the “3Ps” sustainability target (profit/people/planet). The developed process technology was successfully validated under cGMP plant conditions on a scale of 600 kg batch size to supply the fluazuron API (14) across the globe for veterinary use. This process is commercially friendly and environmentally benign. Furthermore, several process-related impurities were identified, synthesized, characterized, and studied for their purging capability. According to the SciFinder database, there are two new impurities (23 and 24), which are structurally similar to the fluazuron API, that could lead to the discovery of new biological applications in both animal and human drug development.
用于合成氟唑隆的热力学控制和工业可行的伸缩工艺
Fluazuron N-[(4-氯-3-[3-氯-5-(三氟甲基)吡啶-2-基]氧基苯基]氨基甲酰基]-2,6-二氟苯甲酰胺(14)是一种值得注意的抗寄生虫兽药,属于苯甲酰苯基脲衍生物,是一类几丁质合成抑制剂。目前,符合现行法规要求(尤其是纯度和杂质含量)的商业规模合成方法尚未成熟。因此,要生产和供应符合所有标准的氟脲或任何药物物质,必须要有一套稳健且可持续的生产工艺。在这项工作中,通过对瓶颈异氰酸酯中间体(20)进行稳健的原位规程,描述了一种安全、可扩展、经济和可持续的生产工艺,从而生产出纯度高达 99.5% HPLC 纯度和 78% 总产率的氟脲脲活性药物成分 (API)。这一大规模 GMP 生产工艺是通过实施 DoE 工具和绿色化学原则(如工艺质量强度评估 (PMI))以及 "3R "原则(减少/再利用/再循环)来建立的,以实现 "3Ps "可持续发展目标(利润/人类/地球)。所开发的工艺技术已在 600 公斤批量的 cGMP 工厂条件下成功验证,可向全球供应兽用氟唑隆原料药 (14)。该工艺对商业和环境无害。此外,还鉴定、合成、表征了几种与工艺相关的杂质,并研究了它们的净化能力。根据 SciFinder 数据库,有两种新杂质(23 和 24)在结构上与氟脲原料药相似,这两种杂质可能会导致在动物和人类药物开发中发现新的生物应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


