Veliparib: Analytical Tools and Process Design Strategies for the Control of Mutagenic Impurities and Other Drug Substance Critical Quality Attributes
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
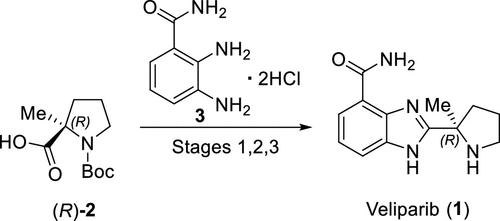
(R)-Veliparib (ABT-888) is a poly(ADP-ribose)polymerase (PARP) inhibitor that is being investigated for the treatment of a broad spectrum of oncology indications, including BRCA1/2-mutated breast cancer and other solid tumors. The (R)-veliparib process consists of three stages utilizing two proposed regulatory starting materials, (R)-Boc-2-methylproline and 2,3-diaminobenzamide dihydrochloride, with two isolated intermediates. The drug substance control strategy, which was established based on a combination of analytical tools and uniquely designed manufacturing process and unit operations, provides robust controls for mutagenic and other impurities and ensures that (R)-veliparib drug substance consistently meets all critical quality attributes (CQAs) and acceptance criteria. The purpose of this article is to provide details of how the (R)-veliparib control strategy for the selected CQAs was cross-functionally developed using analytical measurement tools and specially designed unit operations.
Veliparib:控制突变杂质和其他药物关键质量属性的分析工具和工艺设计策略
(R)-Veliparib(ABT-888)是一种多(ADP-核糖)聚合酶(PARP)抑制剂,目前正在研究用于治疗多种肿瘤适应症,包括 BRCA1/2 突变乳腺癌和其他实体瘤。(R)-veliparib工艺包括三个阶段,利用两种拟议的管制起始原料(R)-Boc-2-甲基脯氨酸和2,3-二氨基苯甲酰胺二盐酸盐以及两种分离的中间体。该药物物质控制策略是在分析工具和独特设计的生产工艺及单元操作相结合的基础上制定的,可对诱变剂和其他杂质进行严格控制,确保 (R)-veliparib 药物物质始终符合所有关键质量属性 (CQA) 和验收标准。本文旨在详细介绍如何利用分析测量工具和专门设计的单元操作,针对选定的 CQA 跨职能制定 (R)-veliparib 控制策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


