Benzofuroxan‐Based Energetic Materials with Alternating Nitro and Hydroxyl Groups: Synthesis, Characterization, and Energetic Properties
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
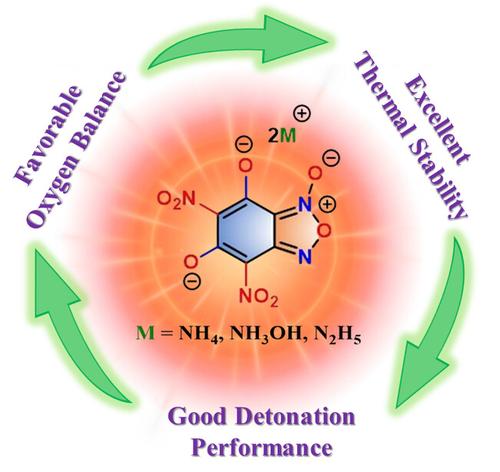
Due to its good stability, density and oxygen balance, the benzofuroxan fused ring framework has attracted particular attention in the field of high energy density materials. The planar structure of the benzofuroxan facilitates the straightforward derivatization with explosophores and contributes to molecular stability. In this work, a benzofuroxan scaffold was utilized to develop a highly dense energetic material, namely 5,7‐dihydroxy‐4,6‐dinitrobenzo[c][1,2,5]oxadiazole 1‐oxide (DHDNBF). The successive inclusion of explosophores like nitro (−NO
具有交替硝基和羟基的苯并呋喃基高能材料:合成、表征和能量特性
由于具有良好的稳定性、密度和氧平衡性,苯并呋喃融合环框架在高能量密度材料领域受到特别关注。苯并呋喃的平面结构有利于与爆炸物直接衍生,并有助于提高分子的稳定性。在这项研究中,我们利用苯并呋喃支架开发了一种高能量密度材料,即 5,7-二羟基-4,6-二硝基苯并[c][1,2,5]恶二唑 1-氧化物(DHDNBF)。在苯并呋喃上相继加入硝基(-NO2)等爆炸性官能团和羟基(-OH)等氧化性官能团后,DHDNBF 的密度(ρ=1.91 g cm-3)和氧平衡(6.20 %)都非常可观(2)。此外,DHDNBF 上的羟基还能形成双阳离子高能盐 3-7,从而进一步改善整体性能。高能盐 3、4 和 5 的密度明显提高,从 1.84(5)到 1.87(3)克/厘米-3 不等,并具有接近零或等于零的良好氧平衡。与中性盐 DHDNBF 相比,所有高能盐(3-7)的热稳定性都有明显改善。高能盐 4(Dv=8459 m s-1,P=32.10 GPa)和 5(Dv=8539 m s-1,P=30.37 GPa)具有良好的高能性能,可与 LLM-105 等著名炸药(Dv=8560 m s-1,P=33.4 GPa)相媲美。总之,高能盐 4 和 5 的良好特性使它们成为在各种军事和民用领域分别用作苯并呋喃类二级和一级炸药的潜在候选物质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


