PdII/CuI-Cocatalyzed Radical Arylation of gem-Difluoroalkenes Using Arylsulfonyl Chlorides
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A PdII/CuI-cocatalyzed arylation of gem-difluoroalkenes with arylsulfonyl chlorides, affording various defluorinative arylation/1,2-difunctionalized products, was developed. The interception of aryl radicals generated from the reduction of arylsulfonyl chlorides delivers some hypervalent Pd species, which present high reactivities and chemoselectivities toward the defluorinative arylation product formation. Besides, the nature of the electron-deficient Pd metal center is more prone to reductive elimination under acidic conditions, providing an opportunity to explore new reactivates of fluorinated alkenes into more elaborate substructures.
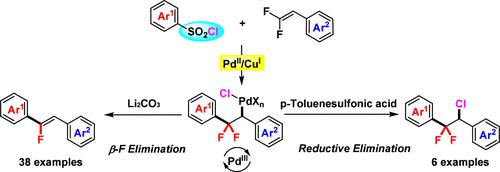
PdII/CuI-Cocalyzed Radical Arylation of gem-Difluoroalkenes Using Arylsulfonyl Chlorides(芳基磺酰氯催化宝石二氟烯烃的芳基化反应
研究人员开发了一种 PdII/CuI 催化的芳基磺酰氯对 gem-difluoroalkenes 的芳基化反应,可得到各种脱氟芳基化/1,2-二官能化产物。拦截芳基磺酰氯还原产生的芳基自由基会产生一些高价钯,这些钯对脱氟芳基化产物的形成具有很高的反应活性和化学选择性。此外,缺电子钯金属中心的性质在酸性条件下更容易发生还原消除,这为探索将氟化烯还原成更复杂亚结构的新反应物提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


