Acceptor End‐functionalization of Naphthalenediimide Bithiophene Oligomers
IF 2.5
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
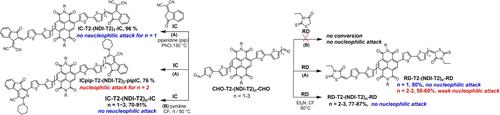
Oligomeric materials combine advantageous properties of both their small molecule and polymeric counterparts. Utilizing oligomers as non‐fullerene acceptors (NFAs) has been shown to be extremely useful for the development of organic solar cells with high efficiency, reproducible performance and long‐term stability. Here we report on two series of synthetically simple acceptor‐terminated oligomers A−T2‐(NDI−T2)n‐A with naphthalene diimide (NDI) and bithiophene (T2) cores up to the trimer (n =1,2,3). Termination of the oligomers is done using the strong acceptors (A) dicyanomethylene‐indanone (IC) and rhodanine (RD). Upon acceptor termination in the presence of piperidine (pip) as base, oligomers with pip‐substituted tricyclic end groups are obtained in high yield. We investigate the effect of oligomer length and acceptor end group on opto‐electronic properties and crystallinity. Both IC‐ and RD‐termination increase electron affinity compared to the parent, non‐functionalized cores. UV‐vis absorption in solution slightly redshifts as the chain length increases without showing a distinct aggregation. Asymmetric termination with hexylphenyl‐substituted indacenodithiophene (IDT) and IC is also possible. All symmetric oligomers show a strong tendency for crystallization, with the oligomer having the tricyclic end group exhibiting the highest melting enthalpy and temperature. The asymmetric IDT−T2‐NDI−T2‐IC oligomer is amorphous.

萘二亚胺双噻吩低聚物的受体端功能化
低聚物材料兼具小分子材料和聚合物材料的优势特性。事实证明,利用低聚物作为非富勒烯受体(NFA)对于开发具有高效率、可重复性能和长期稳定性的有机太阳能电池非常有用。在此,我们报告了两组合成简单的受体端接低聚物 A-T2-(NDI-T2)n-A,其核心为萘二亚胺(NDI)和噻吩(T2),直至三聚体(n =1,2,3)。使用强受体(A)二氰基亚甲基茚酮 (IC) 和罗丹宁 (RD) 终止低聚物。在以哌啶(pip)为碱存在的情况下终止受体时,可以高产率地获得具有哌啶取代的三环末端基团的低聚物。我们研究了低聚物长度和受体末端基团对光电特性和结晶度的影响。与未官能化的母核相比,IC 端基和 RD 端基都能提高电子亲和力。随着链长的增加,溶液中的紫外-可见吸收率会略微偏移,但不会出现明显的聚集现象。用己基苯基取代的茚并二噻吩(IDT)和 IC 进行不对称终止也是可行的。所有对称低聚物都显示出强烈的结晶趋势,其中具有三环末端基团的低聚物显示出最高的熔化焓和熔化温度。不对称的 IDT-T2-NDI-T2-IC 低聚物是无定形的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


