Optimization Strategies for Cathode Materials in Lithium–Oxygen Batteries
IF 14
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Developing high energy density, low-cost, and safe batteries remains a constant challenge that not only drives technological innovation but also holds the potential to transform human lifestyles. Although lithium-ion batteries have been widely adopted, their theoretical energy density is nearing its limit. Consequently, there is an urgent need to explore and investigate other battery systems with higher power capacities to propel technological advancements in this field. In this context, metal–oxygen batteries have attracted considerable interest because of their exceptionally high theoretical energy densities. Among the various metal–oxygen batteries, lithium–oxygen (Li–O2) batteries stand out for their highest thermodynamic equilibrium potential (∼2.96 V) and greatest theoretical specific energy (∼3500 Wh kg–1), positioning them as a promising avenue for future energy storage advancements. Over the past few decades, global scientists have conducted extensive research into the electrochemical reaction mechanisms, material sciences, and system designs of Li–O2 batteries (LOBs), achieving numerous significant breakthroughs. Despite these remarkable advancements, research on LOBs is still in its infancy, confronting numerous unresolved critical issues. First, the deposition of Li2O2 on the electrode surface severely hinders further electrochemical reactions, resulting in actual discharge capacities that are far below theoretical values. Second, the kinetics of the oxygen electrode reactions are relatively slow, failing to meet high power demands and inducing severe polarization phenomena, which significantly reduces energy efficiency. Last, byproducts generated during the charge/discharge process lead to the degradation of electrode materials and electrolytes, markedly shortening the cycle life of the battery. The rational design of efficient and durable catalysts for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is considered one of the most effective strategies for overcoming the aforementioned obstacles. In this Account, we summarize the major electronic modulation strategies for developing efficient cathode catalysts, including structural design, composite material construction, surface and interface engineering, and heteroatom doping. First, specific methods to enhance catalyst performance through optimizing material morphology and structural design are discussed. Then, the construction of composite materials is presented to highlight the synergistic effects of various components in improving battery performance. Next, surface and interface engineering, which could regulate charge transfer and reaction activity, is outlined. Finally, the function of heteroatom doping in enhancing catalytic activity and stability by modifying the electronic structure of catalysts is summarized. Building on the optimization of the performance and reliability of each component in LOBs, the outlook for enhancing the overall electrochemical performance of these batteries is presented. We believe that this Account will inspire the development of effective and stable cathode catalysts for Li–air batteries and foster the practical application of this promising energy storage technology in the future.
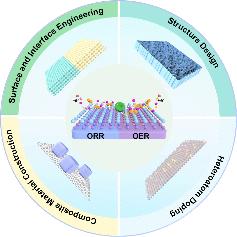
锂氧电池阴极材料的优化策略
开发高能量密度、低成本和安全的电池仍然是一项持续的挑战,它不仅推动着技术创新,而且有可能改变人类的生活方式。尽管锂离子电池已被广泛采用,但其理论能量密度已接近极限。因此,迫切需要探索和研究其他具有更高功率容量的电池系统,以推动该领域的技术进步。在此背景下,金属氧电池因其超高的理论能量密度而备受关注。在各种金属氧电池中,锂-氧(Li-O2)电池以其最高的热力学平衡电位(∼2.96 V)和最大的理论比能量(∼3500 Wh kg-1)而脱颖而出,成为未来能源储存技术发展的一个前景广阔的途径。过去几十年来,全球科学家对锂离子电池(LOB)的电化学反应机理、材料科学和系统设计进行了广泛研究,取得了多项重大突破。尽管取得了这些令人瞩目的进展,但有关锂离子电池的研究仍处于起步阶段,面临着许多尚未解决的关键问题。首先,Li2O2 在电极表面的沉积严重阻碍了进一步的电化学反应,导致实际放电容量远远低于理论值。其次,氧电极反应的动力学过程相对缓慢,无法满足高功率需求,并引发严重的极化现象,从而显著降低了能量效率。最后,充放电过程中产生的副产物导致电极材料和电解液降解,明显缩短了电池的循环寿命。合理设计高效耐用的氧还原反应(ORR)和氧进化反应(OER)催化剂被认为是克服上述障碍的最有效策略之一。在本开户绑定手机领体验金中,我们总结了开发高效阴极催化剂的主要电子调节策略,包括结构设计、复合材料构建、表面和界面工程以及杂原子掺杂。首先,讨论了通过优化材料形态和结构设计来提高催化剂性能的具体方法。然后,介绍了复合材料的构造,以突出各种成分在提高电池性能方面的协同作用。接着,概述了可调节电荷转移和反应活性的表面和界面工程。最后,总结了杂原子掺杂通过改变催化剂的电子结构来提高催化活性和稳定性的作用。在优化 LOB 中每个组件的性能和可靠性的基础上,展望了提高这些电池整体电化学性能的前景。我们相信,该开户绑定手机领体验金将推动锂空气电池有效而稳定的正极催化剂的开发,并促进这一前景广阔的储能技术在未来的实际应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


