Silver-Tungsten induced codeposition: Influence of pH and carboxylic acid form in a DMH-based electrolyte
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
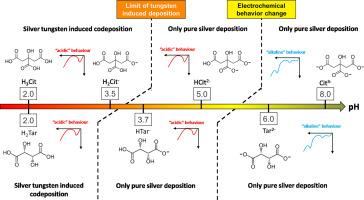
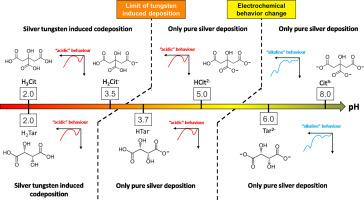
Silver-tungsten coatings were successfully electrodeposited on platinum and copper substrates from a non-toxic 5.5-dimethylhydantoin electrolyte at low pH and two different carboxylic acid forms: citrate or tartrate. Tungsten contents remain at low levels compared to former works, but close to the values considered as optimal for functional properties without having to recourse to controversial substances such as thiourea. Electrochemical studies by linear sweep voltammetry allow to distinguish two typical behaviors and give interesting insight into the induced codeposition mechanism. Silver-tungsten codeposition only occurred at pH 2.0 and 3.5 using citrates and at pH 2.0 using tartrates, corresponding to the forms H3Cit, H2Cit-2, and H2Tar, respectively. No silver-tungsten reduction was possible with less than two protonated carboxyl groups on either tartrate or citrate ions. Separate silver and tungsten lattice are both present in the resulting alloy. Grain and crystallite sizes were observed by SEM. XPS investigations show that for our low W content alloys, silver is found to be metallic whereas tungsten is present in oxide form. The carbon peaks and also gaps between peaks when N is absent indicate that citrates are present in the coating, unlike DMH.
银-钨诱导的共沉积:基于 DMH 的电解液中 pH 值和羧酸形式的影响
在低 pH 值和两种不同的羧酸形式(柠檬酸盐或酒石酸盐)下,使用无毒的 5.5-二甲基海因电解液在铂和铜基底上成功电沉积了银钨涂层。与以前的研究相比,钨的含量仍然较低,但已接近功能特性的最佳值,无需使用硫脲等有争议的物质。通过线性扫描伏安法进行的电化学研究可以区分两种典型的行为,并对诱导的共沉积机制提出了有趣的见解。使用柠檬酸盐时,只有在 pH 值为 2.0 和 3.5 时才会发生银-钨共沉积;使用酒石酸盐时,只有在 pH 值为 2.0 时才会发生银-钨共沉积,分别对应于 H3Cit、H2Cit-2 和 H2Tar 三种形式。当酒石酸根离子或柠檬酸根离子上的质子化羧基少于两个时,银-钨无法还原。生成的合金中存在独立的银晶格和钨晶格。扫描电镜观察到了晶粒和结晶尺寸。XPS 研究表明,对于我们的低 W 含量合金,银是金属性的,而钨则以氧化物形式存在。碳峰和峰间间隙(当不含 N 时)表明涂层中存在柠檬酸盐,这与 DMH 不同。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


