Lithium anode stability enhanced by micro-potentials from spontaneous polarization in BaTiO3 films
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Lithium metal is widely recognized as an ideal anode material due to its high specific capacity and low redox potential. However, challenges such as severe lithium dendrite growth have significantly impeded its practical applications. Herein, we introduce a BaTiO3 (BTO) ferroelectric thin film into the lithium anode. During the charge/discharge cycles, the BTO film generates a micro-potential in the direction opposite to the applied electric field, owing to its spontaneous polarization. The micro-potential effectively reduces the electric field gradient, thereby suppressing inhomogeneous nucleation and the growth of lithium dendrites. Consequently, the incorporation of the BTO ferroelectric thin film significantly enhances the cycling performance of the batteries. The half cells demonstrate stable operation after more than 100 cycles at high capacity of 4 mAh cm−2. Furthermore, the LiFePO4 (LFP) full cell delivers a specific capacity of 146.6 mAh g−1 after 300 cycles. This work demonstrates a promising strategy for the development of lithium batteries with improved longevity and enhanced capacity.
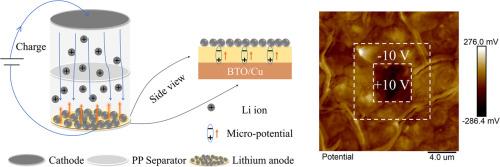
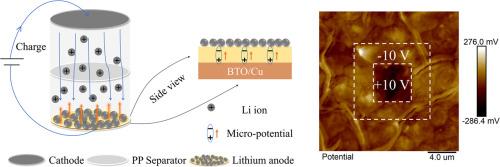
氧化钡薄膜自发极化产生的微电势增强了锂阳极稳定性
金属锂因其高比容量和低氧化还原电位而被公认为理想的正极材料。然而,严重的锂枝晶生长等挑战极大地阻碍了它的实际应用。在此,我们将 BaTiO3 (BTO) 铁电薄膜引入锂负极。在充放电循环过程中,BTO 薄膜会因自发极化作用而产生与外加电场方向相反的微电势。微电势能有效降低电场梯度,从而抑制不均匀成核和锂枝晶的生长。因此,BTO 铁电薄膜的加入大大提高了电池的循环性能。在 4 mAh-cm-2 的高容量条件下,半电池经过 100 多个循环后仍能稳定运行。此外,磷酸铁锂(LFP)全电池在循环 300 次后,比容量达到 146.6 mAh-g-1。这项工作为开发寿命更长、容量更大的锂电池展示了一种前景广阔的战略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


