Cryo-electron tomography reveals how COPII assembles on cargo-containing membranes
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
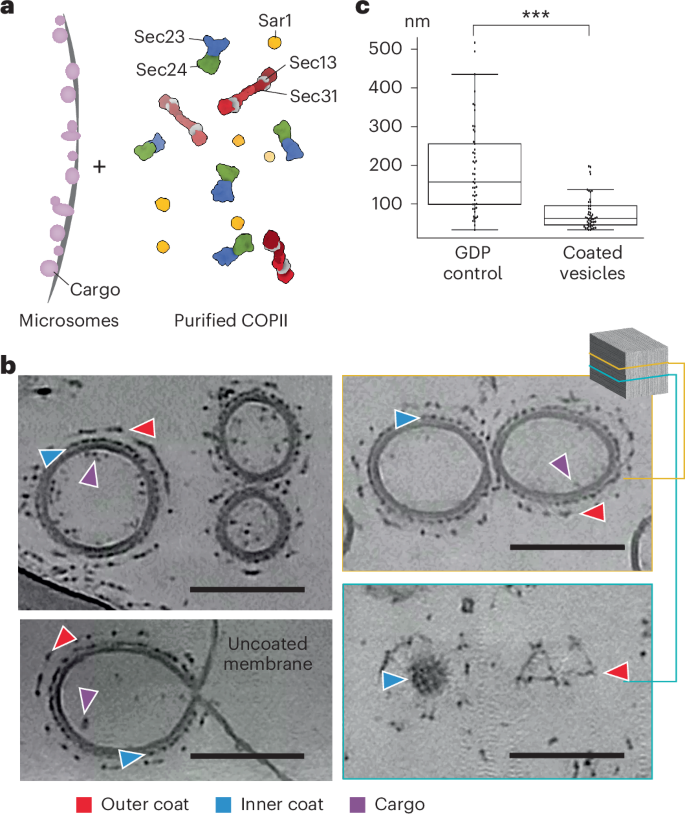
Proteins traverse the eukaryotic secretory pathway through membrane trafficking between organelles. The coat protein complex II (COPII) mediates the anterograde transport of newly synthesized proteins from the endoplasmic reticulum, engaging cargoes with a wide range of size and biophysical properties. The native architecture of the COPII coat and how cargo might influence COPII carrier morphology remain poorly understood. Here we reconstituted COPII-coated membrane carriers using purified Saccharomyces cerevisiae proteins and cell-derived microsomes as a native membrane source. Using cryo-electron tomography with subtomogram averaging, we demonstrate that the COPII coat binds cargo and forms largely spherical vesicles from native membranes. We reveal the architecture of the inner and outer coat layers and shed light on how spherical carriers are formed. Our results provide insights into the architecture and regulation of the COPII coat and advance our current understanding of how membrane curvature is generated. The authors reconstituted coat protein complex II vesicles from native membranes and used cryo-electron tomography and subtomogram averaging to reveal the arrangement of inner and outer coat layers on cargo-containing coated vesicles.

低温电子断层扫描揭示 COPII 如何在含货物的膜上组装
蛋白质通过细胞器之间的膜运输穿越真核生物的分泌途径。衣壳蛋白复合物 II(COPII)介导新合成蛋白质从内质网的逆向运输,并与具有不同大小和生物物理特性的货物接触。人们对 COPII 外壳的原生结构以及货物如何影响 COPII 载体形态仍知之甚少。在这里,我们使用纯化的酿酒酵母蛋白和细胞衍生的微粒体作为原生膜源,重组了 COPII 涂层膜载体。通过使用低温电子断层扫描和子图平均法,我们证明了 COPII 涂层能结合货物,并从原生膜中形成大体呈球形的囊泡。我们揭示了内外衣层的结构,并阐明了球形载体是如何形成的。我们的研究结果为 COPII 外壳的结构和调控提供了见解,并推进了我们目前对膜曲率如何产生的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


