N-glycosylation facilitates the activation of a plant cell-surface receptor
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
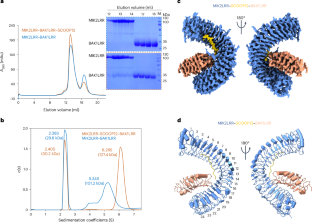
Plant receptor kinases (RKs) are critical for transmembrane signalling involved in various biological processes including plant immunity. MALE DISCOVERER1-INTERACTING RECEPTOR-LIKE KINASE 2 (MIK2) is a unique RK that recognizes a family of immunomodulatory peptides called SERINE-RICH ENDOGENOUS PEPTIDEs (SCOOPs) and activates pattern-triggered immunity responses. However, the precise mechanisms underlying SCOOP recognition and activation of MIK2 remain poorly understood. Here we present the cryogenic electron microscopy structure of a ternary complex consisting of the extracellular leucine-rich repeat (LRR) of MIK2 (MIK2LRR), SCOOP12 and the extracellular LRR of the co-receptor BAK1 (BAK1LRR) at a resolution of 3.34 Å. The structure reveals that a DNHH motif in MIK2LRR plays a critical role in specifically recognizing the highly conserved SxS motif of SCOOP12. Furthermore, the structure demonstrates that N-glycans at MIK2LRRAsn410 directly interact with the N-terminal capping region of BAK1LRR. Mutation of the glycosylation site, MIK2LRRN410D, completely abolishes the SCOOP12-independent interaction between MIK2LRR and BAK1LRR and substantially impairs the assembly of the MIK2LRR–SCOOP12–BAK1LRR complex. Supporting the biological relevance of N410-glycosylation, MIK2N410D substantially compromises SCOOP12-triggered immune responses in plants. Collectively, these findings elucidate the mechanism underlying the loose specificity of SCOOP recognition by MIK2 and reveal an unprecedented mechanism by which N-glycosylation modification of LRR-RK promotes receptor activation. This study demonstrates a crucial role of N-glycosylation in activating a receptor-like kinase by promoting its interaction with co-receptors.

N-糖基化促进植物细胞表面受体的激活
植物受体激酶(RKs)是跨膜信号传导的关键,涉及包括植物免疫在内的各种生物过程。雄性发现者1-互作受体样激酶2(MIK2)是一种独特的RK,它能识别称为ERINE-RICH ENDOGENOUS PEPTIDEs(SCOOPs)的免疫调节肽家族,并激活模式触发的免疫反应。然而,人们对 SCOOP 识别和 MIK2 激活的确切机制仍然知之甚少。在这里,我们以 3.34 Å 的分辨率展示了由 MIK2 的胞外富含亮氨酸的重复序列 (LRR)、SCOOP12 和共受体 BAK1 的胞外 LRR (BAK1LRR) 组成的三元复合物的低温电子显微镜结构。该结构揭示了 MIK2LRR 中的 DNHH 基序在特异性识别 SCOOP12 高度保守的 SxS 基序方面起着关键作用。此外,该结构还表明 MIK2LRRAsn410 上的 N-聚糖直接与 BAK1LRR 的 N 端封顶区相互作用。糖基化位点 MIK2LRRN410D 的突变完全取消了 MIK2LRR 和 BAK1LRR 之间不依赖于 SCOOP12 的相互作用,并大大影响了 MIK2LRR-SCOOP12-BAK1LRR 复合物的组装。支持 N410-糖基化生物学相关性的是,MIK2N410D 会大大损害植物中 SCOOP12 触发的免疫反应。总之,这些发现阐明了 MIK2 识别 SCOOP 的松散特异性机制,并揭示了 LRR-RK 的 N-糖基化修饰促进受体活化的一种前所未有的机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


