Discovery of 4-(4-(3-(1-(2-(piperidin-1-yl)ethyl)-1H-benzo[d]imidazole-2-yl)isoxazol-5-yl)phenyl)morpholine as a novel c-Myc inhibitor against lung cancer in vitro and in vivo
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
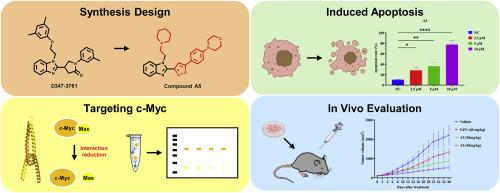
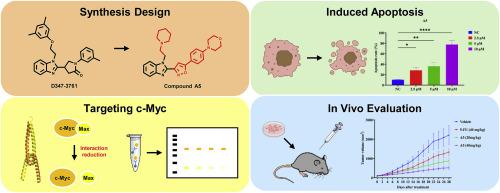
The critical role of c-Myc as a driving factor in the development and progression of lung cancer establishes it as a pivotal target for anti-lung cancer therapeutic research. In our previous study, we reported on the discovery of D347–2761, a novel small-molecule inhibitor that specifically targets the unstable domain of c-Myc and disrupts the c-Myc/Max heterodimer. To enhance targeted therapies further, we conducted an extensive structural analysis and designed a series of innovative benzimidazole derivatives. The cytotoxic activities of these compounds were assessed using the CCK-8 assay, revealing that compound A1 displayed IC50 values of 6.32 μM and 11.39 μM against the A549 and NCI–H1299 lung cancer cell lines, respectively, while compound A5 exhibited IC50 values of 4.08 μM and 7.86 μM against the same cell lines. Our findings revealed that compounds A1 and A5 exhibited potent anticancer activity by disrupting the interaction between c-Myc and Max proteins, leading to the downregulation of c-Myc protein levels and induction of apoptosis through apoptotic pathways. Notably, compound A5 demonstrated superior inhibitory capacity compared to other compounds tested. Furthermore, in a syngeneic tumor model, compound A5 exhibited excellent efficacy with a tumor growth inhibition rate reaching up to 76.4 %, accompanied by a significant reduction in c-Myc protein expression levels. Therefore, compound A5 holds promise as a potential agent for targeting c-Myc in anti-lung cancer therapy.
发现 4-(4-(3-(1-(2-(哌啶-1-基)乙基)-1H-苯并[d]咪唑-2-基)异恶唑-5-基)苯基)吗啉作为一种新型 c-Myc 体外和体内肺癌抑制剂
c-Myc在肺癌的发生和发展过程中起着关键的驱动作用,这使它成为抗肺癌治疗研究的关键靶点。在之前的研究中,我们报道了一种新型小分子抑制剂 D347-2761的发现,它能特异性地靶向 c-Myc 的不稳定结构域并破坏 c-Myc/Max 异二聚体。为了进一步提高靶向治疗的效果,我们进行了广泛的结构分析,并设计出一系列创新的苯并咪唑衍生物。利用 CCK-8 试验评估了这些化合物的细胞毒性活性,结果显示化合物 A1 对 A549 和 NCI-H1299 肺癌细胞株的 IC50 值分别为 6.32 μM 和 11.39 μM,而化合物 A5 对相同细胞株的 IC50 值分别为 4.08 μM 和 7.86 μM。我们的研究结果表明,化合物 A1 和 A5 通过破坏 c-Myc 和 Max 蛋白之间的相互作用,导致 c-Myc 蛋白水平下调,并通过细胞凋亡途径诱导细胞凋亡,从而显示出强大的抗癌活性。值得注意的是,与其他测试化合物相比,化合物 A5 表现出更强的抑制能力。此外,在合成肿瘤模型中,化合物 A5 表现出卓越的疗效,肿瘤生长抑制率高达 76.4%,同时 c-Myc 蛋白表达水平显著降低。因此,化合物 A5有望成为抗肺癌治疗中靶向 c-Myc 的潜在药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


