Discovery of 5-Nitro-N-(3-(trifluoromethyl)phenyl) Pyridin-2-amine as a Novel Pure Androgen Receptor Antagonist against Antiandrogen Resistance
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The transformation of clinical androgen receptor (AR) antagonists into agonists driven by AR mutations poses a significant challenge in treating prostate cancer (PCa). Novel anti-AR therapeutics combating mutation-induced resistance are required. Herein, by combining structure-based virtual screening and biological evaluation, a high-affinity agonist E10 was first discovered. Then guided by the representative conformation of State 1 at the free energy landscape, the structural optimization of E10 was performed, and pure AR antagonists EL15 (IC50 = 0.94 μM) and EF2 (IC50 = 0.30 μM) were successfully identified. Both can antagonize wild-type and variant drug-resistant ARs. Therein, EF2 demonstrated potent inhibition of the AR pathway and effectively suppressed tumor growth in a C4–2B xenograft mouse model following oral administration. Further molecular dynamics simulation and mutagenesis studies revealed atomic insights into the mode of action of EF2 which may serve as a novel lead compound for developing therapeutics against AR-driven PCa.
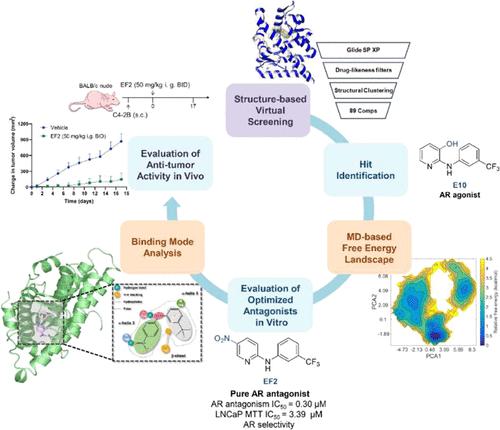
发现 5-硝基-N-(3-(三氟甲基)苯基)吡啶-2-胺作为新型纯雄激素受体拮抗剂可对抗雄激素抗药性
临床上,雄激素受体(AR)拮抗剂会因AR突变而转化为激动剂,这给前列腺癌(PCa)的治疗带来了巨大挑战。需要新型抗 AR 治疗药物来对抗突变引起的耐药性。在此,通过结合基于结构的虚拟筛选和生物学评估,首先发现了一种高亲和力激动剂 E10。随后,以自由能谱中具有代表性的状态 1 构象为指导,对 E10 进行了结构优化,并成功鉴定出纯 AR 拮抗剂 EL15(IC50 = 0.94 μM)和 EF2(IC50 = 0.30 μM)。两者都能拮抗野生型和变异型耐药 AR。其中,EF2 对 AR 通路具有强效抑制作用,在 C4-2B 异种移植小鼠模型中口服后可有效抑制肿瘤生长。进一步的分子动力学模拟和诱变研究揭示了 EF2 的作用模式,可作为开发 AR 驱动的 PCa 治疗药物的新型先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


