Impact of Particle Size on the Vapor-Phase Oxidative Coupling of Methanol and Dimethylamine over Palladium–Gold Nanoparticles
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
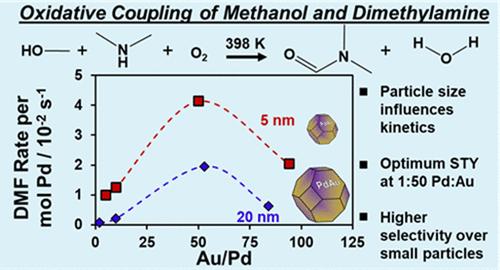
Oxidative coupling of methanol and dimethylamine in the presence of O2 in the vapor phase over dilute Pd in Au bimetallic catalysts occurs via the dissociation of O2 on Pd and selective oxidation of methanol on Au. Here, we synthesize a series of silica-supported PdAu alloy nanoparticle catalysts of varied Pd:Au ratios with ∼5 nm particle diameter and show that these catalysts have increased selectivity to dimethylformamide across all Pd:Au ratios (∼95%), distinct from observations over larger PdAu nanoparticles (∼15–25 nm diameter) of similar Pd:Au ratios. Small monometallic Pd particles are more selective than large monometallic Pd particles, and small Au nanoparticles are reactive and selective for oxidative coupling (while large Au nanoparticles are inactive). Rates per surface metal atom were similar over PdAu nanoparticles of all sizes and increased monotonically with increasing Pd content for the small nanoparticles. Apparent reaction kinetics demonstrate distinct apparent methanol reaction order and apparent activation energy relative to those reported over larger nanoparticles of similar Pd:Au ratios. Unlike larger PdAu nanoparticles, the rate of dimethylformamide formation is not promoted by cofed water over small PdAu nanoparticles. The results of the kinetic studies are used to propose a series of elementary steps, derive a plausible rate expression, and regress rate and equilibrium constants. These results suggest high coverages of surface methoxy species and low coverages of adsorbates derived from dimethylamine. Taken together, these results demonstrate the sensitivity of the rates, selectivities, and kinetics of oxidative coupling reactions to the size of bimetallic nanoparticles.
颗粒尺寸对钯金纳米颗粒上甲醇和二甲胺气相氧化偶联的影响
在稀钯金双金属催化剂上,气相中存在 O2 的甲醇和二甲胺通过 O2 在钯上的解离和甲醇在金上的选择性氧化发生氧化偶联。在这里,我们合成了一系列二氧化硅支撑的 PdAu 合金纳米颗粒催化剂,其 Pd:Au 比各不相同,颗粒直径为 5 nm,结果表明这些催化剂在所有 Pd:Au 比下对二甲基甲酰胺的选择性都有所提高(95%),这与在类似 Pd:Au 比的较大 PdAu 纳米颗粒(直径为 15-25 nm)上观察到的结果不同。小的单金属钯颗粒比大的单金属钯颗粒更具选择性,而小的金纳米颗粒对氧化偶联具有反应性和选择性(而大的金纳米颗粒则不活跃)。在各种尺寸的 PdAu 纳米粒子上,每个表面金属原子的速率相似,并且随着小纳米粒子中 Pd 含量的增加而单调增加。表观反应动力学表明,与所报道的具有相似钯金比的较大纳米粒子相比,甲醇反应的表观顺序和表观活化能有所不同。与较大的 PdAu 纳米粒子不同,在较小的 PdAu 纳米粒子上,二甲基甲酰胺的形成速率并没有受到共注水的促进。动力学研究的结果用于提出一系列基本步骤,推导出合理的速率表达式,并回归速率常数和平衡常数。这些结果表明,表面甲氧基物质的覆盖率较高,而二甲胺衍生吸附剂的覆盖率较低。总之,这些结果证明了氧化偶联反应的速率、选择性和动力学对双金属纳米粒子大小的敏感性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


