Endothelial NMMHC IIA dissociation from PAR1 activates the CREB3/ARF4 signaling in thrombin-mediated intracerebral hemorrhage
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
There is an urgent need for cerebroprotective interventions to improve the suboptimal outcomes with intracerebral hemorrhage (ICH). Despite the important role of nonmuscle myosin heavy chain IIA (NMMHC IIA) in the blood–brain barrier (BBB), its function in ICH remains unclear.Objectives
The objective of this study is to explore how NMMHC IIA functions in ICH and to evaluate the effectiveness of targeting NMMHC IIA as a treatment for ICH.Methods
We firstly examined the protein expression of NMMHC IIA in clinical patients and animal models with ICH. The function of NNMMHC IIA was then corroborated by using overexpress or knockdown NMMHC IIA specifically in ECs mice and pBMECs. In addition, we explored protein interacts with NMMHC IIA and signaling pathways after ICH by LC-MS/MS and transcriptomics analysis with an emphasis on the function of PAR1 and the CREB3/ARF4 signaling pathway, and validated them in three kind of animal models. To support the clinical translation of our results, we targeted NMMHC IIA to bicalutamide selected from a library of marketed drugs and examined to validate its ameliorative effect on ICH.Results
We observed an upregulation of endothelial NMMHC IIA in the brain following the onset of ICH in both patients and mice, while inhibited NMMHC ⅡA improved ICH induced by thrombin, warfarin or tissue plasminogen activator (tPA) after ischemic stroke. Mechanistically, the head domain of NMMHC IIA interacted with protease-activated receptor 1 (PAR1) at the 380–430 aa region and subsequently dissociated and activated the CREB3/ARF4 signaling pathway. We found that bicalutamide and blebbistatin could bind to NMMHC IIA and effectively protect mice from thrombin-mediated ICH.Conclusion
The findings indicated that NMMHC IIA dissociated from PAR1 and activated CREB3/ARF4 pathway, which aggravated BBB damage induced by thrombin. This suggested that NMMHC IIA was a novel potential therapeutic target for BBB-related diseases.
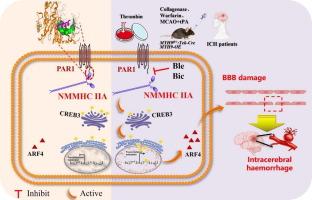
内皮 NMMHC IIA 与 PAR1 分离可激活凝血酶介导的脑内出血中的 CREB3/ARF4 信号传导
引言目前迫切需要脑保护干预措施来改善脑内出血(ICH)的不良预后。尽管非肌肉肌球蛋白重链 IIA(NMMHC IIA)在血脑屏障(BBB)中发挥着重要作用,但它在 ICH 中的功能仍不清楚。方法我们首先检测了 NMMHC IIA 在临床患者和 ICH 动物模型中的蛋白表达。我们首先检测了临床患者和 ICH 动物模型中 NNMMHC IIA 蛋白的表达,然后通过在小鼠 ECs 和 pBMECs 中特异性过表达或敲除 NNMMHC IIA 来证实 NNMMHC IIA 的功能。此外,我们还通过 LC-MS/MS 和转录组学分析探讨了与 NMMHC IIA 相互作用的蛋白质以及 ICH 后的信号通路,重点研究了 PAR1 和 CREB3/ARF4 信号通路的功能,并在三种动物模型中进行了验证。结果我们观察到患者和小鼠发生 ICH 后脑内皮 NMMHC IIA 上调,而抑制 NMMHC ⅡA 可改善缺血性卒中后凝血酶、华法林或组织纤溶酶原激活剂(tPA)诱导的 ICH。从机理上讲,NMMHC ⅡA的头部结构域在380-430 aa区域与蛋白酶激活受体1(PAR1)相互作用,随后解离并激活CREB3/ARF4信号通路。研究结果表明,NMMHC IIA 与 PAR1 分离并激活 CREB3/ARF4 通路,从而加重了凝血酶诱导的 BBB 损伤。这表明,NMMHC IIA 是治疗 BBB 相关疾病的潜在新靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


