Concerted Proton-Coupled Electron Transfer by Mo5+/Mo6+ Reversible Transformation for CO2 Photoreduction with Nearly 100% CH4 Selectivity
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Regulation of the proton-coupled electron transfer (PCET) process to avoid the unbalanced proton and electron regions on the reduction active sites is key to dictating product selectivity in a photocatalytic CO2 reduction reaction. Here, we show that reversible Mo5+/Mo6+ as a mediator can regulate the proton and electron transfer process at the Bi2MoO6 nanosheet/In2O3 microtube (BI) catalyst. The formed concerted proton-coupled electron transfer enables a champion solar-to-methane efficiency of 0.15%, resulting in nearly 100% CH4 selectivity and a competitive CH4 yield of 46.37 μmol g–1 h–1 in pure water. The experiments, together with theoretical calculations, clearly validate that In sites as H2O oxidation centers provide protons, and the regulation of protons and electrons by using Mo sites forms approximate electroneutral proton/electron pairs, which are conjointly transferred to Bi sites as CO2 adsorption/reduction centers, thus achieving precise hydrogenation on Bi sites for binding of the *CH3O key intermediate to form CH4.
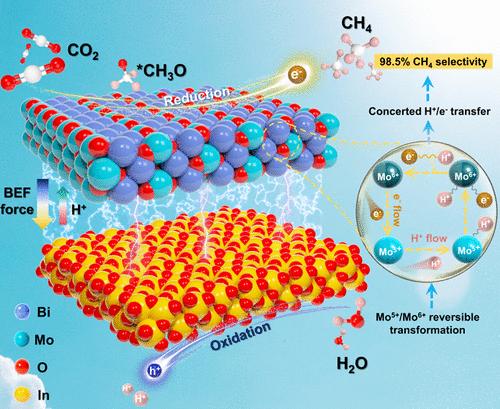
通过 Mo5+/Mo6+ 可逆转化实现协同质子耦合电子转移,以接近 100% 的 CH4 选择性进行 CO2 光还原
调节质子耦合电子转移(PCET)过程以避免还原活性位点上质子和电子区域的不平衡,是决定光催化二氧化碳还原反应中产物选择性的关键。在这里,我们展示了可逆的 Mo5+/Mo6+ 作为介质可以调节 Bi2MoO6 纳米片/In2O3 微管(BI)催化剂上的质子和电子转移过程。所形成的质子耦合电子传递使太阳能转化为甲烷的效率达到了 0.15%,从而使甲烷的选择性接近 100%,在纯水中的甲烷产率高达 46.37 μmol g-1 h-1。实验和理论计算清楚地验证了 In 位点作为 H2O 氧化中心提供质子,通过使用 Mo 位点调节质子和电子,形成近似电中性的质子/电子对,这些质子/电子对同时转移到作为 CO2 吸附/还原中心的 Bi 位点,从而在 Bi 位点上实现精确氢化,结合 *CH3O 关键中间产物形成 CH4。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


