Influence of alumina on the performance of Ag/ZnO based catalysts for carbon dioxide hydrogenation
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We study silver-zinc oxide type catalysts with and without the addition of alumina and perform structural analysis and activity tests for the hydrogenation of carbon dioxide. Adding alumina has a dispersing effect on the zinc oxide without structurally altering the silver phase. An alumina-surface enriched ZnO/Al2O3 phase is observed with an increased surface reducibility. Ag/ZnO has a high selectivity towards carbon monoxide (63 ± 12 %) and methane (24 ± 3 %) and low selectivity towards methanol (13 ± 0.5 %). Operando infrared (SSITKA-FTIR) and mass spectrometric product detection indicate methane formation via an adsorbed carbon monoxide (COads) intermediate. The selectivity changes gradually with increasing alumina content, up to 80 ± 3 % toward methanol, and 20 ± 4 % carbon monoxide without methane detection, combined with a tripling of the space time yield to 0.65 ± 0.02 mmolMeOH**h−1 at 250 °C and 30 bar. Kinetic analysis suggests that the selectivity change originates from hindering the CO-pathway, while the formate pathway leading to methanol remains active.
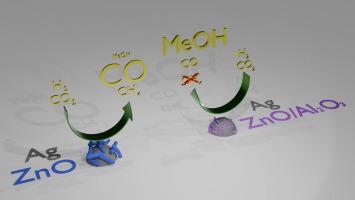
氧化铝对基于 Ag/ZnO 的二氧化碳氢化催化剂性能的影响
我们研究了添加和不添加氧化铝的银氧化锌型催化剂,并进行了结构分析和二氧化碳氢化活性测试。添加氧化铝对氧化锌有分散作用,但不会改变银相的结构。观察到氧化铝表面富集的氧化锌/Al2O3 相,其表面还原性增加。氧化铝/氧化锌对一氧化碳(63 ± 12 %)和甲烷(24 ± 3 %)的选择性较高,而对甲醇(13 ± 0.5 %)的选择性较低。操作红外(SSITKA-FTIR)和质谱产品检测表明,甲烷是通过吸附的一氧化碳(COads)中间体形成的。随着氧化铝含量的增加,选择性逐渐发生变化,在 250 °C 和 30 bar 条件下,对甲醇的选择性高达 80 ± 3 %,而对一氧化碳的选择性为 20 ± 4 %,但未检测到甲烷,同时空间产率增加了两倍,达到 0.65 ± 0.02 mmolMeOH*gcat-1*h-1。动力学分析表明,选择性变化源于一氧化碳途径受阻,而甲酸途径导致甲醇仍然活跃。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


