Targeting glycolysis for neuroprotection in early LPS-induced neuroinflammation
IF 3.7
Q2 IMMUNOLOGY
引用次数: 0
Abstract
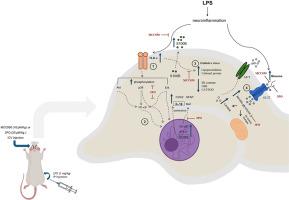
Neuroinflammation is a pathophysiological feature of numerous neurological and psychiatric disorders. The immune response in the central nervous system, driven by microglia and astrocytes, leads to metabolic reprogramming towards aerobic glycolysis, a phenomenon known as the Warburg effect. The control of metabolic reprogramming via immunomodulation may represent a potential therapeutic target for providing protection against neuroinflammation, which contributes to neuronal dysfunction and death in several neurological disorders. For this purpose, we investigated putative neuroprotective effects of the downregulation of aerobic glycolysis using the 3PO inhibitor, and the downregulation of neuroinflammation using MCC950, in the early LPS-induced neuroinflammation model. The LPS-induced shift towards glycolysis, inflammatory and glial changes (IL-1β, NF-κB, COX2, Iba1, GFAP) were reversed by 3PO, which improved animal behavior. Additionally, MCC950 (an NLRP3 inhibitor) downregulated TLR4/Akt/p38 MAPK/NF-κB/STAT3 signaling, expressions of COX2 and IL-1β, and the astrocyte reactivity (decreasing GFAP) induced by early neuroinflammation, resulting in low glucose uptake. Our data support the occurrence of the Warburg effect during early neuroinflammation and suggest potential new approaches for the treatment of brain injury, given the role of neuroinflammation in such events.
以糖酵解为靶点,在早期 LPS 诱导的神经炎症中提供神经保护
神经炎症是许多神经和精神疾病的病理生理特征。在小胶质细胞和星形胶质细胞的驱动下,中枢神经系统的免疫反应会导致新陈代谢向有氧糖酵解方向重编程,这种现象被称为沃伯格效应。通过免疫调节控制新陈代谢重编程可能是一种潜在的治疗靶点,可用于防止神经炎症,因为神经炎症会导致多种神经系统疾病的神经元功能障碍和死亡。为此,我们研究了在早期 LPS 诱导的神经炎症模型中,使用 3PO 抑制剂下调有氧糖酵解和使用 MCC950 下调神经炎症的潜在神经保护作用。3PO 逆转了 LPS 诱导的糖酵解转变、炎症和神经胶质变化(IL-1β、NF-κB、COX2、Iba1、GFAP),从而改善了动物的行为。此外,MCC950(一种 NLRP3 抑制剂)可下调 TLR4/Akt/p38 MAPK/NF-κB/STAT3 信号传导、COX2 和 IL-1β 的表达以及早期神经炎症诱导的星形胶质细胞反应性(GFAP 降低),从而导致低葡萄糖摄取。我们的数据支持在早期神经炎症过程中出现沃伯格效应,鉴于神经炎症在此类事件中的作用,我们提出了治疗脑损伤的潜在新方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain, behavior, & immunity - health
Biological Psychiatry, Behavioral Neuroscience
CiteScore
8.50
自引率
0.00%
发文量
0
审稿时长
97 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


