Rapid adsorptive removal of congo red using Cu/Fe/Al mixed metal oxides obtained from layered double hydroxide nanomaterial: Performance and optimization evaluation using BBD-RSM approach
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
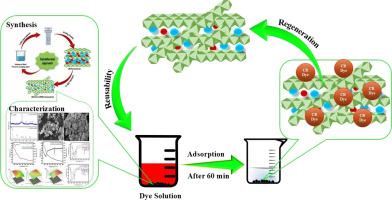
This work utilized a one-pot synthesis method to synthesize Cu/Fe/Al mixed metal oxide (MMO) from layered double hydroxide (LDH) calcination. The adsorption potentials of Cu/Fe/Al LDH and Cu/Fe/Al MMOs were evaluated using Congo Red (CR) aqueous solution. Various advanced techniques were used to characterize the synthesized nanomaterial. XRD confirmed the successful synthesis of trimetallic carbonate intercalated LDH and MMO, while FE-SEM revealed their flaky morphology. EDX and FT-IR analyses supported the dye removal mechanism, showing that MMOs had greater adsorptive removal of CR molecules than LDH. This was primarily due to the MMOs' larger surface area and microporous structure (132 m²/g) which favoured greater adsorption. The adsorption kinetics reveal that the adsorptive removal of CR on LDH and MMOs follows a pseudo-second-order reaction. Moreover, as predicted by Freundlich the maximum adsorptive removal was obtained by LDH-derived MMO (96%) which was higher than the LDH (90%). The thermodynamics indicates that adsorption was exothermic and thus more favorable at room temperature. In addition, the Box-Behnken Design (BBD) was adopted for Response Surface Methodology (RSM) due to its simplicity in analysis and effectiveness in systematically evaluating the impact of pH, dosage of adsorbent, dye concentration, contact period, and temperature variables. The pHzpc of 7.9 indicated that lower pH enhances adsorption, and a +5.1 mV charge on the MMO surface, measured by zeta potential, supported electrostatic interaction with the anionic dye. Lastly, LDH and LDH-derived MMO were regenerated using 1 M NaOH and effectively reused for up to 5 cycles. MMOs demonstrated superior reusability compared to LDH for anionic dye removal. Thus, the synthesised Cu/Fe/Al MMO, with its large surface area, offers a superior adsorbent for removing organic pollutants.
利用从层状双氢氧化物纳米材料中获得的 Cu/Fe/Al 混合金属氧化物快速吸附去除刚果红:使用 BBD-RSM 方法进行性能和优化评估
本研究采用一锅合成法,从层状双氢氧化物(LDH)煅烧中合成了铜/铁/铝混合金属氧化物(MMO)。利用刚果红(CR)水溶液评估了 Cu/Fe/Al LDH 和 Cu/Fe/Al MMO 的吸附电位。合成纳米材料的表征采用了多种先进技术。XRD 证实了三金属碳酸盐插层 LDH 和 MMO 的成功合成,而 FE-SEM 则揭示了它们的片状形态。EDX 和 FT-IR 分析表明,MMO 对 CR 分子的吸附去除率高于 LDH。这主要是由于 MMOs 具有更大的比表面积和微孔结构(132 m²/g),有利于更大程度地吸附。吸附动力学表明,LDH 和 MMO 对 CR 的吸附去除遵循假二阶反应。此外,正如 Freundlich 预测的那样,LDH 衍生的 MMO 获得了最大的吸附去除率(96%),高于 LDH(90%)。热力学表明,吸附是放热的,因此在室温下更有利。此外,由于方框-贝肯设计(BBD)分析简单,且能有效系统地评估 pH 值、吸附剂用量、染料浓度、接触时间和温度等变量的影响,因此采用了方框-贝肯设计(BBD)响应面方法(RSM)。pHzpc 值为 7.9 表明,较低的 pH 值会增强吸附作用,通过 zeta 电位测量,MMO 表面的 +5.1 mV 电荷支持与阴离子染料的静电相互作用。最后,用 1 M NaOH 对 LDH 和 LDH 衍生的 MMO 进行再生,可有效重复使用长达 5 个周期。与 LDH 相比,MMO 在去除阴离子染料方面的重复利用率更高。因此,合成的 Cu/Fe/Al MMO 具有较大的表面积,是一种去除有机污染物的优质吸附剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


