Molecular design of −substituted boron difluoride curcuminoids: Tuning luminescence and nonlinear optical properties
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-22
DOI:10.1016/j.jphotochem.2024.116110
引用次数: 0
Abstract
Boron β-diketonates are prospective fluorescent dyes for functional organic materials to new technologies in the fields of chemistry, NLO-optics, photonics and bio-imaging. The novel NIR-to-NIR luminophors – curcuminoids of boron difluoride with different substituents at the central carbon atom (at the γ-position) of the chelate ring 1,7-bis(4′-N,N′-dimethylaminophenyl)-4-organyl-gept-1,6-dien-3,5-dionate of boron difluoride (1–5) were synthesized. Luminescence in solutions, crystals and polymer matrices, photobiological properties and NLO properties in polystyrene (PS) and polymethylmethacrylate (PMMA) were studied. The crystal structure has been determined for 1. For 1–5, in PS film, upon excitation by laser (365 nm), two luminescence bands are observed in the blue (430 nm) and red (650–700 nm) regions of the spectrum. For 1–5, two-photon luminescence is recorded in PS and PMMA films. PS 1 films demonstrate high photostability. The synthesized dyes turned out to be almost non-toxic for HCT116 tumor cells both in a long-term dark experiment (72 h) and after photoexcitation in accordance with absorption maxima in the submicromolar concentration range. The compounds were rapidly accumulated by cells within 3 h, demonstrating cytoplasmic distribution and the potential for use as cellular photoimaging agents. The accumulation of dyes with hydrocarbon γ-substitutes (methyl, iso-propyl, phenyl) after 3 and 24 h is more effective compared to dye 2 (commercial product Cranad-2).
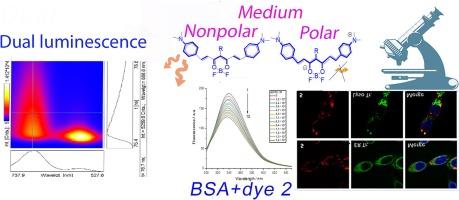
二氟化硼姜黄素的分子设计:调谐发光和非线性光学特性
β-二酮酸硼是一种前景广阔的荧光染料,可用于化学、NLO-光学、光子学和生物成像领域新技术的功能性有机材料。本研究合成了新型近红外到近红外发光体--二氟化硼螯合环 1,7-双(4′-N,N′-二甲氨基苯基)-4-有机烷基-1,6-庚二烯-3,5-二酮酸(1-5)的姜黄素,其中心碳原子(γ 位)上有不同的取代基。研究了溶液、晶体和聚合物基质中的发光、光生物特性以及聚苯乙烯(PS)和聚甲基丙烯酸甲酯(PMMA)中的 NLO 特性。1 的晶体结构已经确定。对于 1-5,在 PS 薄膜中,经激光(365 nm)激发后,在光谱的蓝色(430 nm)和红色(650-700 nm)区域观察到两条发光带。1-5 在 PS 和 PMMA 薄膜中记录到双光子发光。PS 1 薄膜具有很高的光稳定性。在长期黑暗实验(72 小时)和光激发后,根据亚摩尔浓度范围内的吸收最大值,合成的染料对 HCT116 肿瘤细胞几乎无毒。这些化合物在 3 小时内迅速在细胞中积累,显示出细胞质分布和用作细胞光成像剂的潜力。与染料 2(商业产品 Cranad-2)相比,带有碳氢化合物 γ 替代物(甲基、异丙基、苯基)的染料在 3 小时和 24 小时后的累积效果更好。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


