Selenate oxyanion-intercalated NiFeOOH for stable water oxidation via lattice oxygen oxidation mechanism
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
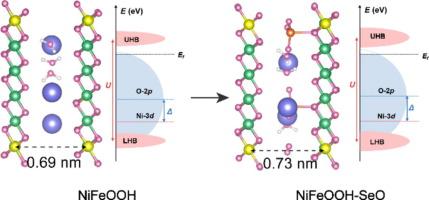
Transition metal-based compounds can serve as pre-catalysts to obtain genuine oxygen evolution reaction (OER) electrocatalysts in the form of oxyhydroxides through electrochemical activation. However, the role and existence form of leached oxygen anions are still controversial. Herein, we selected iron selenite-wrapped hydrated nickel molybdate (denoted as NiMoO/FeSeO) as a pre-catalyst to study the oxyanion effect. It is surprising to find that SeO42− exists in the catalyst in the form of intercalation, which is different from previous studies that suggest that anions are doped with residual elements after electrochemical activation, or adsorbed on the catalyst surface. The experiment and theoretical calculations show that the existence of SeO42− intercalation effectively adjusts the electronic structure of NiFeOOH, promotes intramolecular electron transfer and O–O release, and thus lowers the reaction energy barrier. As expected, the synthesized NiFeOOH-SeO only needs 202 and 285 mV to attain 100 and 1000 mA cm−2 in 1 M KOH. Further, the anion exchange membrane water electrolyzer (AEMWE) consisting of NiFeOOH-SeO anode and Pt/C cathode can reach 1 A cm−2 at 1.70 V and no significant attenuation within 300 h. Our findings provide insights into the mechanism, by which the intercalated oxyanions enhance the OER performance of NiFeOOH, thereby facilitating large-scale hydrogen production through AEMWE.
通过晶格氧氧化机制实现稳定水氧化的硒氧阴离子互掺 NiFeOOH
过渡金属基化合物可作为前催化剂,通过电化学活化以氧氢氧化物的形式获得真正的氧进化反应(OER)电催化剂。然而,浸出氧阴离子的作用和存在形式仍存在争议。在此,我们选择了硒酸铁包裹的水合钼酸镍(记为 NiMoO/FeSeO)作为前催化剂来研究氧阴离子效应。令人惊讶的是,SeO42- 以插层形式存在于催化剂中,这与以往研究认为阴离子是在电化学活化后掺入残余元素或吸附在催化剂表面的观点不同。实验和理论计算表明,SeO42-插层的存在有效地调整了 NiFeOOH 的电子结构,促进了分子内电子转移和 O-O 释放,从而降低了反应能垒。正如预期的那样,在 1 M KOH 中,合成的 NiFeOOH-SeO 只需要 202 和 285 mV 就能达到 100 和 1000 mA cm-2。此外,由 NiFeOOH-SeO 阳极和 Pt/C 阴极组成的阴离子交换膜水电解槽(AEMWE)可在 1.70 V 的电压下达到 1 A cm-2,且在 300 小时内无明显衰减。我们的研究结果深入揭示了插层氧阴离子提高 NiFeOOH 的 OER 性能的机制,从而促进了通过 AEMWE 大规模制氢。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


