Additive effect of Li on electrical property of ZnO passivation layer to control dendritic growth of Zn during recharge processes
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
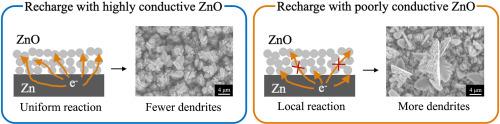
This study investigates the effect of Li+ on the dendritic growth of Zn anodes in the presence of a ZnO passivation layer formed after discharge, with particular attention to the initial recharge process. 0.1 mol dm−3 Li+ effectively suppresses dendrites, while 2 mol dm−3 Li+ addition facilitates the same. The difference in Zn dendrite formation behavior is also indicated by the attenuation tendency of the potential oscillation accompanied by hydrogen evolution reaction during recharge. This is attributed to Li+ concentration dependence of the properties of the ZnO passivation layer formed during Zn anode discharge. Li+ modulates the carrier density of ZnO by altering its crystalline defect characteristics; the carrier density of ZnO with 0.1 mol dm−3 Li+ addition becomes approximately three times as high as that without additive owing to the oxygen vacancies and interstitial zinc that form additional donor level. By contrast, 2 mol dm−3 Li+ reduces the carrier density of ZnO by inducing zinc vacancies to form acceptor levels. The highly conductive ZnO produced by adding 0.1 mol dm−3 Li+ improves the reaction uniformity during recharge, which suppresses dendrite formation. This study provides valuable insight into the mechanisms and control strategies of Zn dendrite growth during the charge-discharge cycling of alkaline Zn rechargeable batteries.
锂对氧化锌钝化层电气性能的添加效应,可在充电过程中控制锌的树枝状生长
本研究探讨了在放电后形成的氧化锌钝化层存在的情况下,Li+对锌阳极树枝状生长的影响,尤其关注初始充电过程。0.1 mol dm-3 Li+ 能有效抑制树枝状生长,而添加 2 mol dm-3 Li+ 则能促进树枝状生长。Zn 树枝状形成行为的差异还表现在充电过程中伴随氢进化反应的电位振荡的衰减趋势。这归因于锌阳极放电过程中形成的氧化锌钝化层的特性与 Li+ 浓度有关。Li+ 通过改变 ZnO 的晶体缺陷特性来调节其载流子密度;添加 0.1 mol dm-3 Li+ 的 ZnO 的载流子密度大约是不添加添加剂的三倍,这是因为氧空位和间隙锌形成了额外的供体水平。相比之下,2 mol dm-3 Li+ 会诱导锌空位形成受体水平,从而降低氧化锌的载流子密度。添加 0.1 mol dm-3 Li+ 生成的高导电性氧化锌改善了充电过程中的反应均匀性,从而抑制了枝晶的形成。这项研究为了解碱性锌充电电池充放电循环过程中锌枝晶的生长机制和控制策略提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


