Prognostic value of tumor-infiltrating lymphocytes in distal extrahepatic bile duct carcinoma
IF 7.1
2区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Background
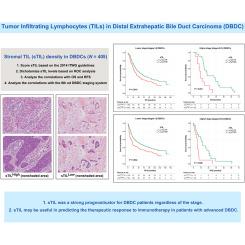
The assessment of tumor-infiltrating lymphocytes (TILs) has led to the development of various immunotherapies beyond their predictive potential in gastrointestinal malignancies. However, the clinicopathologic and prognostic values of TILs have yet to be well elucidated in distal extrahepatic bile duct carcinoma (DBDC).
Patients and methods
We evaluated stromal TILs (sTILs) and intraepithelial TILs (iTILs) in 405 surgically resected DBDCs to analyze their correlations with overall survival (OS) and recurrence-free survival (RFS) and with clinicopathologic parameters according to the eighth edition of the American Joint Committee on Cancer scheme.
Results
High levels of sTIL density (sTILHigh; >5%) and iTIL count (iTILHigh; >3) were found in 245 (61%) and 74 cases (18%), respectively. sTILHigh was more commonly found in larger tumors (P = 0.048) diffusely involving both intra- and extrapancreatic bile ducts (P = 0.013), in tumors with lower T category (P = 0.002), and in tumors without pancreatic (P = 0.003) or duodenal invasion (P < 0.001). iTILHigh was associated with tumors with papillary or nodular growth pattern (P < 0.001) without perineural invasion (P = 0.006). Both sTILHigh and iTILHigh significantly predicted better OS (P = 0.009 and 0.036, respectively) and RFS (P = 0.003 and 0.026, respectively). sTIL consistently provided prognostic predictability in OS, even when tested with different quantitative cut-offs and prognostically stratified OS (P = 0.006) and RFS (P = 0.005) on multivariate analysis. The survival benefit of sTILHigh persisted regardless of the stage in both OS (P = 0.010 for lower stages I and II and P = 0.001 for higher stages III and IV) and RFS (P = 0.004 and 0.025 for lower- and higher-stage tumors, respectively).
Conclusions
sTILs were superior to iTILs in predicting survival, and it was shown to be a strong prognosticator for DBDC patients regardless of the stage. The utility of sTILs may extend beyond prognostication to aid in predicting therapeutic responses in DBDC patients.
远端肝外胆管癌中肿瘤浸润淋巴细胞的预后价值
背景对肿瘤浸润淋巴细胞(TILs)的评估已促成了各种免疫疗法的开发,并超越了其在胃肠道恶性肿瘤中的预测潜力。然而,TILs在远端肝外胆管癌(DBDC)中的临床病理和预后价值尚未得到很好的阐明。患者和方法我们评估了405例手术切除的DBDC的基质TILs(sTILs)和上皮内TILs(iTILs),根据美国癌症联合委员会第八版方案分析了它们与总生存期(OS)和无复发生存期(RFS)以及临床病理参数的相关性。结果分别在 245 例(61%)和 74 例(18%)中发现了高水平的 sTIL 密度(sTILHigh; >5%)和 iTIL 计数(iTILHigh; >3)。sTILHigh 更常见于较大的肿瘤(P = 0.048)、弥漫性累及胰内和胰外胆管的肿瘤(P = 0.iTILHigh与肿瘤的乳头状或结节状生长模式(P< 0.001)相关,但无神经周围侵犯(P = 0.006)。sTILHigh和iTILHigh都能显著预测较好的OS(P = 0.009和0.036,分别为0.003和0.026)和RFS(P = 0.003和0.026,分别为0.009和0.036)。即使采用不同的定量临界值进行测试,并在多变量分析中对OS(P = 0.006)和RFS(P = 0.005)进行预后分层,sTIL仍能持续预测OS的预后。无论分期如何,sTILHigh 的生存获益在 OS(I 期和 II 期较低时 P = 0.010,III 期和 IV 期较高时 P = 0.001)和 RFS(低期和高期肿瘤分别为 P = 0.004 和 0.025)方面均持续存在。sTILs 的效用可能超出预后,有助于预测 DBDC 患者的治疗反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ESMO Open
Medicine-Oncology
CiteScore
11.70
自引率
2.70%
发文量
255
审稿时长
10 weeks
期刊介绍:
ESMO Open is the online-only, open access journal of the European Society for Medical Oncology (ESMO). It is a peer-reviewed publication dedicated to sharing high-quality medical research and educational materials from various fields of oncology. The journal specifically focuses on showcasing innovative clinical and translational cancer research.
ESMO Open aims to publish a wide range of research articles covering all aspects of oncology, including experimental studies, translational research, diagnostic advancements, and therapeutic approaches. The content of the journal includes original research articles, insightful reviews, thought-provoking editorials, and correspondence. Moreover, the journal warmly welcomes the submission of phase I trials and meta-analyses. It also showcases reviews from significant ESMO conferences and meetings, as well as publishes important position statements on behalf of ESMO.
Overall, ESMO Open offers a platform for scientists, clinicians, and researchers in the field of oncology to share their valuable insights and contribute to advancing the understanding and treatment of cancer. The journal serves as a source of up-to-date information and fosters collaboration within the oncology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


