Abnormal adsorption of lithium on the graphene surface of graphene/dT(H)-MoS2 heterostructures
IF 2.1
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The graphene/MoS2 heterostructures (Gr/MoS2) exhibit excellent performance for ion batteries, such as superior stability and cyclicity for ion battery storage, and have great potentials for other applications. Lithium (Li) adsorption on/in Gr/MoS2 heterostructures exhibits advanced properties and interesting phenomena, as well as the phase engineering of MoS2. However, unified understanding for the different adsorption behaviors remains lacking, although fully understanding to the adsorption behaviors is of vital importance for their applications. In the current work, the Li adsorptions on the Gr surface of Gr/dT(H)-MoS2 heterostructures are systematically analyzed based on density functional theory calculations, and highlight the differences between Gr/H-MoS2 and Gr/dT-MoS2 for Li adsorption. To fully understand the adsorption behaviors, we perform detailed analyses from four interrelated aspects: 1) Electrostatic interactions from detailed Bader charge analysis, 2) charge density difference Δρ(z), 3) energy-level alignment between Li and the band edges of Gr, dT-, and H-MoS2, and 4) the resulted interface dipoles. We find that partial electrons in Li can pass through Gr to H-MoS2 and the origin is attributed to the weak electronic-shielding of Gr (even weaker than H-MoS2). All of the above extended analysis not only enables us to understand the abnormal adsorption of Li on the Gr surface of Gr/dT(H)-MoS2 heterostructures, but also helps guide the selection of ion battery materials. Moreover, we extend the discussion of Li adsorption to other alkali metal atoms with smaller work functions (such as: Na and K). Our work not only provides understanding to the abnormal adsorption of Li on the Gr surface of Gr/dT(H)-MoS2 heterostructures, but also helps guide the selection of ion battery materials. So, the insights from this study are important for their related applications. This paper reveals and explains an interesting abnormal adsorption phenomenon of lithium on van der Waals heterostructures of graphene and different phases of MoS2.The conclusion and insights from this work is not limited to Li (applicable at least to Na and K, also), and hence our work is helpful for establishing the surface-adsorption mechanisms of ion batteries.
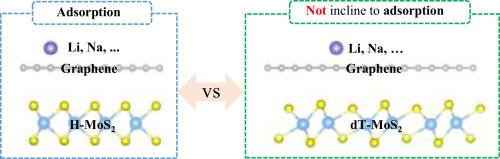
石墨烯/dT(H)-MoS2 异质结构的石墨烯表面对锂的异常吸附
石墨烯/MoS2 异质结构(Gr/MoS2)在离子电池方面表现出卓越的性能,例如在离子电池存储方面具有超强的稳定性和循环性,在其他应用方面也具有巨大的潜力。锂(Li)在 Gr/MoS2 异质结构上的吸附表现出先进的性能和有趣的现象,以及 MoS2 的相工程。然而,尽管充分了解吸附行为对其应用至关重要,但对不同吸附行为仍缺乏统一的认识。在目前的工作中,基于密度泛函理论计算,系统分析了 Gr/dT(H)-MoS2 异质结构的 Gr 表面对锂的吸附,并强调了 Gr/H-MoS2 和 Gr/dT-MoS2 对锂吸附的差异。为了充分理解吸附行为,我们从四个相互关联的方面进行了详细分析:1) 通过详细的 Bader 电荷分析得出的静电相互作用;2) 电荷密度差 Δρ(z);3) Li 与 Gr、dT- 和 H-MoS2 带边之间的能级排列;以及 4) 由此产生的界面偶极子。我们发现,Li 中的部分电子可以穿过 Gr 到达 H-MoS2,其原因在于 Gr 的弱电子屏蔽(甚至比 H-MoS2 更弱)。上述所有扩展分析不仅使我们能够理解锂在 Gr/dT(H)-MoS2 异质结构的 Gr 表面的异常吸附,而且有助于指导离子电池材料的选择。此外,我们还将锂吸附的讨论扩展到了功函数较小的其他碱金属原子(如 Na 和 K)。我们的工作不仅有助于理解锂在 Gr/dT(H)-MoS2 异质结构的 Gr 表面的异常吸附,还有助于指导离子电池材料的选择。因此,本研究的见解对其相关应用具有重要意义。本文揭示并解释了锂在石墨烯和不同相 MoS2 的范德华异质结构上的一种有趣的异常吸附现象。这项工作的结论和见解不仅限于锂(至少也适用于 Na 和 K),因此我们的工作有助于建立离子电池的表面吸附机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


