Light-driven enhancement of CO2 hydrogenation via nickel loading and oxygen vacancy formed on indium oxide
IF 4.3
3区 材料科学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Photothermal catalysis is an efficient approach for CO2 conversion. Herein, the light-driven enhancement of CO2 hydrogenation via nickel loading and oxygen vacancy formed on indium oxide was investigated. The 5.17 wt% Ni/In2O3-x catalyst exhibited a CO2 conversion of ∼32 % with a CO production rate of 14.4 mmol gcat−1 h−1 under visible light irradiation at 300 °C. The photothermal catalytic activity was four times that of the thermocatalytic reaction and over twice that of In2O3-x under the same photothermal conditions. Structural characterization methods, including XRD, TEM, H2-TPR, CO2-TPD, and XPS, confirmed the importance of suppressing the over-reduction of the Ni–In2O3-x interface, to maintain both metallic Ni and oxygen vacancies for the photothermal CO2 conversion. UV–vis absorption, PL, XPS, and DFT calculation results verified the positive effect of the Ni loading and the formation of oxygen vacancies on enhancing visible light absorption and photoelectron-hole separation of Ni/In2O3-x, thus providing more photoelectrons for CO2 conversion to CO. Additionally, the increasing metallic Ni phase and the regeneration of oxygen vacancies induced by visible light irradiation were discovered. The synergistic effect of the Ni loading and oxygen vacancies on In2O3-x plays a key role in enhancing the photothermal catalytic CO2 hydrogenation.
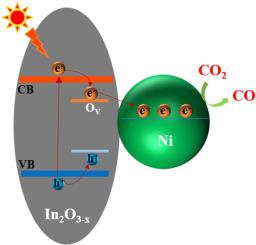
通过镍负载和氧化铟上形成的氧空位实现光驱动的二氧化碳氢化增强效应
光热催化是一种高效的二氧化碳转化方法。本文研究了通过镍负载和氧化铟上形成的氧空位来提高光驱动的二氧化碳加氢。在 300 °C 的可见光照射下,5.17 wt% Ni/In2O3-x 催化剂的 CO2 转化率为 ∼32 %,CO 生成率为 14.4 mmol gcat-1 h-1。在相同的光热条件下,光热催化活性是热催化反应的四倍,是 In2O3-x 的两倍多。包括 XRD、TEM、H2-TPR、CO2-TPD 和 XPS 在内的结构表征方法证实了抑制 Ni-In2O3-x 界面过度还原的重要性,以保持金属镍和氧空位,促进光热转化 CO2。紫外-可见吸收、聚光、XPS 和 DFT 计算结果验证了镍负载和氧空位的形成对增强镍/In2O3-x 的可见光吸收和光电子-空穴分离的积极作用,从而为 CO2 转化为 CO 提供更多的光电子。此外,还发现了金属镍相的增加和可见光照射诱导的氧空位再生。In2O3-x 上的镍负载和氧空位的协同效应在提高光热催化 CO2 加氢过程中发挥了关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
2.50%
发文量
605
审稿时长
40 days
期刊介绍:
The Journal of Physics and Chemistry of Solids is a well-established international medium for publication of archival research in condensed matter and materials sciences. Areas of interest broadly include experimental and theoretical research on electronic, magnetic, spectroscopic and structural properties as well as the statistical mechanics and thermodynamics of materials. The focus is on gaining physical and chemical insight into the properties and potential applications of condensed matter systems.
Within the broad scope of the journal, beyond regular contributions, the editors have identified submissions in the following areas of physics and chemistry of solids to be of special current interest to the journal:
Low-dimensional systems
Exotic states of quantum electron matter including topological phases
Energy conversion and storage
Interfaces, nanoparticles and catalysts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


