Synthesis of 1,3,5-trisubstituted 1,2,4-triazoles enabled by a gold-catalyzed three-component reaction
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Valuable fully substituted 1,2,4-triazoles are obtained via a gold-catalyzed three-component reaction involving ethyl diazoacetate, aryldiazonium salts, and nitriles. The reaction proceeds under mild conditions, is regioselective, and allows the introduction of mono- and di-substituted aryl rings at the ortho, meta, and para positions. Mechanistic evidence suggests the participation of Au(III) species as the active catalysts.
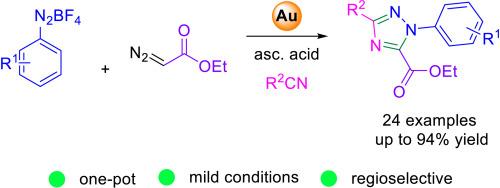
通过金催化的三组分反应合成 1,3,5-三取代的 1,2,4-三唑
通过金催化的重氮乙酸乙酯、芳基重氮盐和腈的三组分反应,获得了有价值的全取代 1,2,4 三唑。该反应在温和的条件下进行,具有区域选择性,可在正位、偏位和对位引入单取代和二取代芳基环。机理证据表明,Au(III) 物种是活性催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


