Quantitative analysis of three bioactive components of Biancaea decapetala extracts in rat plasma and RAW264.7 cells using UPLC-MS/MS and its application to comparative pharmacokinetics in normal and diseased states
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Biancaea decapetala (Roth) O.Deg. (Fabaceae), traditionally utilized by the Hmong for treating rheumatoid arthritis (RA), has its pharmacokinetic behavior under disease conditions largely unexplored. In view of this, a UPLC-MS/MS method was established for the determination of protosappanin B (PTB), protosappanin B-3-O-β-D-glucoside (PTD), and 3-deoxysappanchalcone (3-DSC), key bioactive components of the herb, in rat plasma and RAW264.7 cells to explore the effect of disease state on the pharmacokinetic profiles changes of these three components in vitro and in vivo. These components were detected using multiple reaction monitoring (MRM) process in positive and negative mode. Each calibration curve had a high R2 value of > 0.99. The intra- and inter-day precisions of PTD, PTB, 3-DSC were all < 15 %, and accuracy ranged from 85 % to 115 %. The RSD values pertaining to stability, recovery, matrix effect, and stability remained below 15.0 %. It was successfully applied for the investigation of the pharmacokinetics of these three components in rat plasma and RAW264.7 cells after administration of Biancaea decapetala extracts (BDE). In rat pharmacokinetic experiments, significant differences were observed in the AUC(0-t), MRT(0-t), and Clz/F values of PTD, PTB, 3-DSC between adjuvant-induced arthritis (AA) and normal rats. In cellular pharmacokinetic experiments, comparison with the normal group revealed increased AUC(0-t) and MRT(0-t) for these three components in the LPS-induced inflammatory cell model, along with decreased Clz/F, which was consistent with in vivo experimental outcomes. These findings suggest an increased absorption rate and a decreased elimination rate of the three components of BDE in AA rats and inflammatory cells, indicating a potential alteration in the rate and extent of drug metabolism. This study provided a theoretical reference for further clarification of its pharmacodynamic basis.
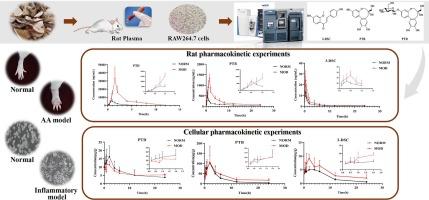
利用 UPLC-MS/MS 对大鼠血浆和 RAW264.7 细胞中的扁柏提取物的三种生物活性成分进行定量分析,并将其应用于正常和疾病状态下的药代动力学比较
Biancaea decapetala (Roth) O.Deg.(豆科)是苗族传统用于治疗类风湿性关节炎(RA)的药物,但其在疾病条件下的药代动力学行为在很大程度上尚未得到研究。有鉴于此,本研究建立了一种 UPLC-MS/MS 方法,用于测定大鼠血浆和 RAW264.7 细胞中的原苏木素 B(PTB)、原苏木素 B-3-O-β-D-葡萄糖苷(PTD)和 3-脱氧苏木查耳酮(3-DSC)这三种草药的主要生物活性成分,以探讨疾病状态对这三种成分在体外和体内药代动力学特征变化的影响。这些成分采用多反应监测(MRM)工艺在正负模式下进行检测。每条校准曲线的 R2 值均高达 0.99。PTD、PTB和3-DSC的日内和日间精确度均为15%,准确度为85%至115%。稳定性、回收率、基质效应和稳定性的 RSD 值均低于 15.0 %。该方法成功地应用于研究大鼠血浆和 RAW264.7 细胞在服用扁蓄草提取物(BDE)后这三种成分的药代动力学。在大鼠药代动力学实验中,PTD、PTB、3-DSC 的 AUC(0-t)、MRT(0-t)和 Clz/F 值在佐剂诱导的关节炎(AA)大鼠和正常大鼠之间存在显著差异。在细胞药代动力学实验中,与正常组比较发现,在 LPS 诱导的炎症细胞模型中,这三种成分的 AUC(0-t) 和 MRT(0-t) 值增加,Clz/F 值降低,这与体内实验结果一致。这些研究结果表明,BDE 的三种成分在 AA 大鼠和炎症细胞中的吸收率增加,消除率降低,表明药物代谢的速度和程度可能发生了改变。这项研究为进一步阐明其药效学基础提供了理论参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


