Synergistic leaching of lithium from clay-type lithium ore using sulfuric acid and oxalic acid
IF 5.3
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
With the escalating market demand for lithium, the development and efficient utilization of lithium resources have become crucial. This study introduced a method for leaching lithium from raw clay-type lithium ores using a composite sulfuric acid oxalic acid system. The experimental results revealed that the optimal conditions for the leaching process included roasting temperature of 600 °C, sulfuric acid concentration of 0.8 mol/L, liquid-solid ratio of 5 mL/g, leaching temperature of 90 °C, leaching duration of 90 min, and oxalic acid dosage of 2 g. Under these conditions, the leaching efficiency of lithium reached 93.45 %. The structural changes during the lithium leaching process and leaching mechanism were analyzed by XRD, SEM, TOF-SIMS. It was found that the minerals after mixed-acid leaching showed a loose morphology, a decrease in the average particle size, and a significant increase in the specific surface area as well as the pore volume, leading to improved lithium leaching efficiency. Furthermore, the mechanism underlying the mixed-acid leaching of lithium from clay-type lithium ores was explored. According to this mechanism, sulfuric acid first dissociated H+, which disrupted the mineral structure, allowing further destruction by oxalic acid. During this process, Li+ was continuously replaced by H+ and reacted with C2O42− dissociated from oxalic acid to form water-soluble Li2C2O4.
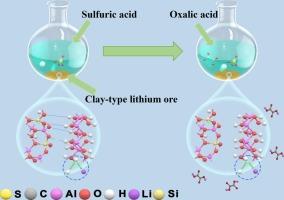
使用硫酸和草酸协同浸出粘土型锂矿中的锂
随着市场对锂需求的不断增长,锂资源的开发和高效利用变得至关重要。本研究介绍了一种利用复合硫酸草酸体系从粘土型锂原矿中浸出锂的方法。实验结果表明,浸出过程的最佳条件包括焙烧温度 600 ℃、硫酸浓度 0.8 mol/L、液固比 5 mL/g、浸出温度 90 ℃、浸出时间 90 min、草酸用量 2 g。通过 XRD、SEM、TOF-SIMS 分析了锂浸出过程中的结构变化和浸出机理。结果发现,混合酸浸出后的矿物形态疏松,平均粒径减小,比表面积和孔隙率显著增加,从而提高了锂浸出效率。此外,还探讨了粘土型锂矿石混合酸浸出锂的机理。根据这一机制,硫酸首先解离出 H+,从而破坏了矿物结构,使草酸得以进一步破坏。在此过程中,Li+不断被H+取代,并与草酸中解离出的C2O42-反应,形成水溶性的Li2C2O4。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


