Revealing the intrinsic mechanism of the influence of different rings and oxidized structures on the room temperature phosphorescence
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2024-11-01
DOI:10.1016/j.saa.2024.125366
引用次数: 0
Abstract
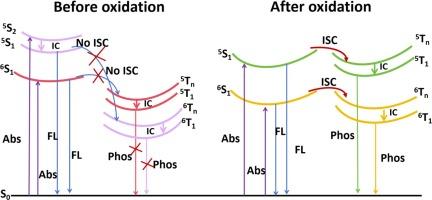
In this work, we used density functional theory (DFT) and time-dependent density functional theory (TD-DFT) methods to study the mechanism of pure organic room temperature phosphorescence emission. The effects on the electronic structure and photochemical properties of thiophene and diketone derivatives with different cyclic and oxidized structures. The result suggests that varying ring configurations and oxidation products significantly influence the photochemical characteristics of thiophene and diketone derivatives. The complex experiences conformational distortion along with the oxidation product, which causes notable alterations in the energy gap and charge density of its frontier molecular orbitals. An oxidation process significantly distorts the molecular structure of the compound, leading to excited singlet and excited triplet states structural similarities. With energy gaps dropping from 0.22 eV to 0.05 eV and spin–orbit coupling constants rising from 0.42 cm−1 to 57.48 cm−1, the excited singlet and excited triplet states share structures and charge distributions that increase the energy level channels appropriate for intersystem crossing. Therefore, this work can provide theoretical support for the design and structural optimization of highly efficient pure organic phosphorescent materials.
揭示不同环和氧化结构对室温磷光影响的内在机制。
在这项工作中,我们采用密度泛函理论(DFT)和时变密度泛函理论(TD-DFT)方法研究了纯有机物室温磷光发射的机理。不同环状结构和氧化结构的噻吩和二酮衍生物对电子结构和光化学性质的影响。结果表明,不同的环构型和氧化产物对噻吩和二酮衍生物的光化学特性有显著影响。随着氧化产物的出现,复合物的构象也发生了畸变,这导致其前沿分子轨道的能隙和电荷密度发生了显著变化。氧化过程极大地扭曲了化合物的分子结构,导致激发单线态和激发三线态结构相似。由于能隙从 0.22 eV 下降到 0.05 eV,自旋轨道耦合常数从 0.42 cm-1 上升到 57.48 cm-1,激发的单重态和激发的三重态具有相同的结构和电荷分布,从而增加了适合系统间交叉的能级通道。因此,这项工作可为高效纯有机磷光材料的设计和结构优化提供理论支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


