Citicoline eluting hydrogels for therapeutic contact lenses intended to treat neurodegenerative diabetic ocular diseases
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
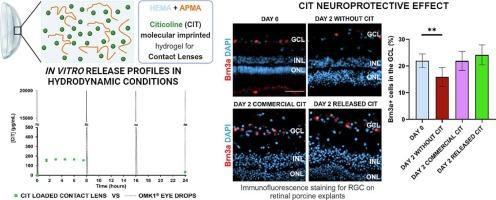
Ophthalmic neurodegenerative diseases related to diabetes, such as glaucoma and retinopathy, are among the major causes of blindness in the world. Citicoline (CIT) in the form of eye drops is currently used for the treatment/prevention of these diseases, which affect the posterior segment of the eye. To ensure the drug penetration into the back of the eye, frequent instillations of highly concentrated drug solutions are required with potential side effects. Drug-loaded soft contact lenses (SCLs) may be an effective alternative to the conventional eye drop treatment, since they may enable a sustained drug release during daily wear, ensuring a higher drug bioavailability, and avoiding drug waste. In this work, one 2-hydroxyethyl methacrylate (HEMA) based hydrogel was functionalised with N-(3-aminopropyl) methacrylamide hydrochloride (APMA), molecularly imprinted with CIT and loaded with the same drug. The material was extensively characterised, in terms of morphology, optical, mechanical, and physical–chemical properties, namely, equilibrium water content, wettability, oxygen and ionic permeability, Young’s modulus, shear deformation, transmittance and refractive index, before and after steam sterilisation. Additionally, the tendency of the material to adsorb proteins of the lachrymal fluid was evaluated and its biocompatibility was assessed by irritation and cytotoxicity assays. Comparison with the non-functionalised and non-imprinted hydrogel showed that the modified hydrogel led to a sustained in vitro release of a much higher amount of CIT than the original one, while keeping typical values for physical–chemical properties of SCLs. The drug-loaded material resisted steam sterilisation and proved to be biocompatible, demonstrating adequate properties to be used in therapeutic SCLs for the prophylaxis/treatment of neurodegenerative diabetic ocular diseases. The neuroprotective effect of the released drug was confirmed with tests using porcine retinal explants.
用于治疗神经退行性糖尿病眼病的治疗性隐形眼镜的胞二磷胆碱洗脱水凝胶。
与糖尿病有关的眼部神经退行性疾病,如青光眼和视网膜病变,是全球失明的主要原因之一。目前,滴眼液形式的胞二磷胆碱(CIT)被用于治疗/预防这些影响眼球后段的疾病。为确保药物渗透到眼球后部,需要频繁滴入高浓度的药物溶液,这可能会产生副作用。载药软性隐形眼镜(SCL)可能是传统眼药水治疗的有效替代品,因为它可以在日常佩戴过程中持续释放药物,确保更高的药物生物利用度,并避免药物浪费。在这项研究中,一种基于甲基丙烯酸羟乙酯(HEMA)的水凝胶被 N-(3-氨基丙基)甲基丙烯酰胺盐酸盐(APMA)功能化,并与 CIT 进行分子印迹,同时添加了相同的药物。在蒸汽灭菌前后,对该材料的形态、光学、机械和物理化学特性(即平衡含水量、润湿性、氧和离子渗透性、杨氏模量、剪切变形、透光率和折射率)进行了广泛的表征。此外,还评估了材料吸附泪液蛋白质的倾向,并通过刺激性和细胞毒性实验评估了其生物相容性。与未功能化和未压印的水凝胶相比,改性水凝胶在体外持续释放的 CIT 量远高于原始水凝胶,同时还保持了 SCL 物理化学特性的典型值。这种载药材料可耐受蒸汽灭菌,并具有良好的生物相容性,足以用于预防/治疗神经退行性糖尿病眼病的治疗性 SCL。使用猪视网膜外植体进行的测试证实了释放药物的神经保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


