Deep functional measurements of Fragile X syndrome human neurons reveal multiparametric electrophysiological disease phenotype
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
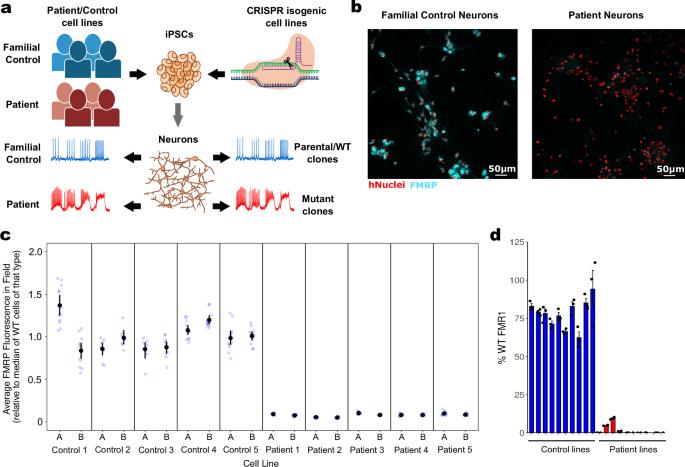
Fragile X syndrome (FXS) is a neurodevelopmental disorder caused by hypermethylation of expanded CGG repeats (>200) in the FMR1 gene leading to gene silencing and loss of Fragile X Messenger Ribonucleoprotein (FMRP) expression. FMRP plays important roles in neuronal function, and loss of FMRP in mouse and human FXS cell models leads to aberrant synaptic signaling and hyperexcitability. Multiple drug candidates have advanced into clinical trials for FXS, but no efficacious treatment has been identified to date, possibly as a consequence of poor translation from pre-clinical animal models to human. Here, we use a high resolution all-optical electrophysiology platform applied to multiple FXS patient-derived and CRISPR/Cas9-generated isogenic neuronal cell lines to develop a multi-parametric FXS disease phenotype. This neurophysiological phenotype was optimized and validated into a high throughput assay based on the amount of FMRP re-expression and the number of healthy neurons in a mosaic network necessary for functional rescue. The resulting highly sensitive and multiparameter functional assay can now be applied as a discovery platform to explore new therapeutic approaches for the treatment of FXS. Deep functional characterization of Fragile X syndrome patient and isogenic neurons using all-optical electrophysiology and machine learning identifies a validated, FMR1-dependent cellular phenotype compatible with high throughput drug screening.
对脆性 X 综合征人类神经元的深度功能测量揭示了多参数电生理疾病表型。
脆性 X 综合征(FXS)是一种神经发育障碍性疾病,由 FMR1 基因中的 CGG 重复序列(大于 200 个)过度甲基化导致基因沉默和脆性 X 信使核糖核蛋白(FMRP)表达缺失引起。FMRP 在神经元功能中发挥着重要作用,在小鼠和人类 FXS 细胞模型中,FMRP 的缺失会导致突触信号异常和过度兴奋。多种候选药物已进入 FXS 临床试验阶段,但迄今为止尚未发现有效的治疗方法,这可能是由于从临床前动物模型到人体的转化效果不佳所致。在这里,我们将高分辨率全光学电生理学平台应用于多个 FXS 患者衍生细胞系和 CRISPR/Cas9 生成的同源神经元细胞系,以建立多参数 FXS 疾病表型。这种神经生理学表型被优化并验证为一种高通量检测方法,其依据是 FMRP 的再表达量以及功能性挽救所需的镶嵌网络中健康神经元的数量。由此产生的高灵敏度和多参数功能测定现在可以作为一个发现平台,用于探索治疗 FXS 的新疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


