Origin of the intermolecular forces that produce donor–acceptor stacks in π-conjugated charge-transfer complexes
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The attraction between π-conjugated planar electron donor and acceptor molecules that form many stable charge-transfer (CT) complexes has been explained by quantum chemical CT interactions, although the fundamental origin remains unclear. Here, we demonstrate the mechanism of CT complex formation by potential energy map analysis for TTF–CA and BTBT–TCNQ, using energy decomposition of intermolecular interaction by symmetry-adapted perturbation theory (SAPT) combined with coupled cluster calculation. We find that the source of attraction between donor and acceptor molecules is ascribed primarily to the dispersion force and also to the electrostatic force. In contrast, the contribution of CT interactions to the attractive forces is minimal. We demonstrate that the highly directional feature of the exchange repulsion force, coupled with the attractive dispersion and electrostatic forces, is crucial in determining the intermolecular arrangements of actual CT crystals. These findings are key for understanding the unique structural and electronic properties of π-conjugated CT complexes. The attraction between π-conjugated planar electron donor and acceptor molecules within charge–transfer (CT) complexes has been explained by quantum chemical CT interactions, but its fundamental origins remain unclear. Here, the authors combine symmetry-adapted perturbation theory with coupled cluster calculations to probe the mechanism of CT complex formation in crystals, finding that dispersion and electrostatic forces are dominant, with significant directional exchange repulsion.
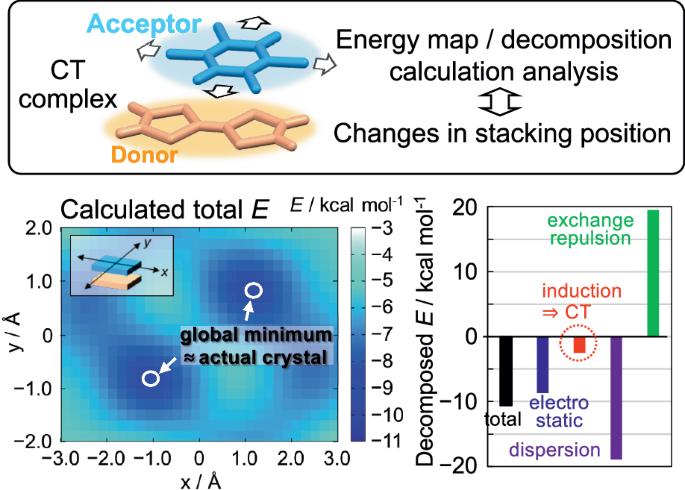
π共轭电荷转移复合物中产生供体-受体堆叠的分子间作用力的起源。
π-共轭平面电子供体分子和受体分子之间的吸引力形成了许多稳定的电荷转移(CT)复合物,量子化学 CT 相互作用解释了这一现象,但其根本原因仍不清楚。在此,我们利用对称性适应扰动理论(SAPT)对分子间相互作用进行能量分解,并结合耦合簇计算,通过对 TTF-CA 和 BTBT-TCNQ 的势能图分析,证明了 CT 复合物的形成机制。我们发现,供体分子和受体分子之间的吸引力主要来自分散力和静电力。相比之下,CT 相互作用对吸引力的贡献微乎其微。我们的研究表明,交换斥力的高方向性特征,加上有吸引力的色散力和静电力,是决定实际 CT 晶体分子间排列的关键。这些发现对于理解π-共轭 CT 复合物的独特结构和电子特性至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


