Rhodium-Catalyzed Enantioselective Ring-Openings of Oxabicyclic Alkenes with Azole Nucleophiles.
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-11-15
Epub Date: 2024-11-06
DOI:10.1021/acs.joc.4c02437
引用次数: 0
Abstract
We report enantioselective ring-openings of oxabicyclic alkenes with azole nucleophiles, generating heterocycle-bearing dihydronaphthalene products. Pyrazoles, triazoles, tetrazoles, and benzo-fused derivatives participate in the ring-opening, with the level of regioselectivity depending on the type and substitution pattern of the heterocyclic partner. Electron-withdrawing azole substituents have a beneficial effect, suppressing the unproductive complexation of a nitrogen with the Rh(I)-bis(phosphine) catalyst.
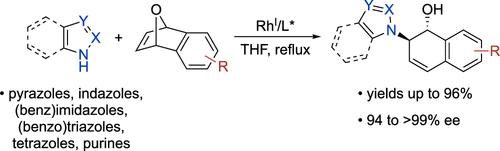
铑催化的羰基双环烯与偶氮唑核亲和剂的对映选择性开环。
我们报告了氧杂双环烯与唑类亲核物的对映选择性开环反应,生成含杂环的二氢萘产物。吡唑、三唑、四唑和苯并融合衍生物参与了开环反应,其区域选择性的高低取决于杂环伙伴的类型和取代模式。吸电子的唑取代基具有有利影响,可抑制氮与 Rh(I)- 双(膦)催化剂的非生产性络合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


