Definite Treatment Delay With Neoadjuvant Chemotherapy and Longitudinal Monitoring by Circulating Tumor DNA for Advanced Cervical Cancer During Pregnancy: A Case Series and Literature Review
Abstract
Background
Previous studies mainly concentrate on neoadjuvant chemotherapy (NACT) for delivery delay in FIGO Stage IB1–IIIB cervical cancer during pregnancy to prevent early preterm delivery while not affecting maternal outcome.
Case
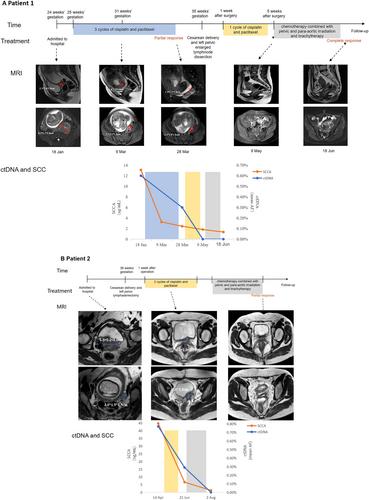
Here, we described two pregnant patients with FIGO Stage IIIC cervical cancer about their diagnosis, treatment, and outcome. Both patients underwent cesarean delivery, left enlarged lymph node dissection, and longitudinal monitoring by circulating tumor DNA. Our study suggested that pregnant patient was completely response to NACT, which was confirmed by ctDNA monitoring, followed by left pelvic enlarged lymph node dissection during cesarean section and adjuvant chemoradiotherapy postpartum. The infant grew normally, without any evidence of chemotherapy-related side effects after delivery.
Conclusion
In pregnant women with advanced cervical cancer, longitudinal ctDNA monitoring might be able to evaluate maternal response to NACT and confirm if delivery delay to optimize fetal outcome would compacting the maternal outcomes or not. Cervical cancer may not transmit across the placental barrier and so it is safe for delayed delivery until fetal maturity in utero during pregnancy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


