Assessment of cerium adsorption potential of phosphoric acid activated biochar in aqueous system: Modelling and mechanistic insights
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Cerium pollution in waterbodies by improper industrial waste disposal is a major concern due to its detrimental impacts on the environment. Therefore, treatment of cerium-contaminated water is inevitable. Hence, this study is focused on the remediation of cerium pollution using phosphoric acid-activated biochar (PPMB) as an adsorbent, synthesized upon pyrolytic activation of palmyra palm male flower-based pristine biochar (PMFB) with H3PO4 at 500 °C. The physico-chemical surface properties of PMFB and PPMB were evaluated through various microscopic and spectroscopic analyses. The key parameters such as biochar dosage, pH, temperature, contact time and initial cerium concentration were optimized as 0.5 g/L, 5.0, 303 K, 180 min and 50 mg/L respectively via batch adsorption. Pseudo-second order kinetic and Toth isotherm are the best-fitted models. The thermodynamic parameters including ΔG◦ (−30.4707 ± 0.7618 kJ/mol at 303 K), ΔH◦ (16.1499 ± 0.78 kJ/mol), and ΔS◦ (153.617 ± 3.8404 J/mol/K) conveying that cerium adsorption onto PPMB was spontaneous, endothermic, and highly disordered at PPMB-bulk adsorption medium interface. Precipitation, electrostatic attraction, and surface complexation are predicted to be the predominant mechanisms for the chosen PPMB-cerium adsorption system. Moreover, cerium phytotoxicity on Vigna radiata explains the real-time applicability and feasibility of cerium adsorption using PPMB. Thus, the key findings of this study specified that the higher adsorption capacity of PPMB (141.3484 ± 6.9856 mg/g) contributed by the incorporated phosphate groups, predominant mesoporosity, SSABET of 230.559 m2/g and anionic surface at a wider pH range (pH>3.08) make PPMB as efficient, economically feasible and environmentally friendly adsorbent for cerium adsorption in aqueous system.
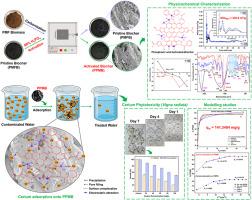
评估磷酸活化生物炭在水体系中的铈吸附潜力:建模与机理认识。
由于工业废物处理不当,水体中的铈污染对环境造成了有害影响,这已成为人们关注的主要问题。因此,对受铈污染的水体进行处理势在必行。因此,本研究的重点是使用磷酸活性生物炭(PPMB)作为吸附剂修复铈污染。PPMB 是棕榈雄花基原始生物炭(PMFB)与 H3PO4 在 500°C 高温下热解活化后合成的。通过各种显微镜和光谱分析,对 PMFB 和 PPMB 的物理化学表面特性进行了评估。通过批量吸附,生物炭用量、pH 值、温度、接触时间和初始铈浓度等关键参数分别优化为 0.5 g/L、5.0、303 K、180 分钟和 50 mg/L。伪二阶动力学和托斯等温线是拟合效果最好的模型。热力学参数包括ΔG◦(-30.4707±0.7618 kJ/mol,303 K 时)、ΔH◦(16.1499±0.78 kJ/mol)和ΔS◦(153.617±3.8404 J/mol/K),表明铈在 PPMB 上的吸附是自发的、内热的,并且在 PPMB-大容量吸附介质界面上高度无序。据预测,沉淀、静电吸引和表面络合是所选 PPMB 铈吸附系统的主要机制。此外,铈对金莲花的植物毒性说明了使用 PPMB 吸附铈的实时适用性和可行性。因此,本研究的主要发现表明,PPMB 的吸附容量较高(141.3484±6.9856 mg/g),这主要归功于其含有的磷酸基团、占主导地位的介孔率、230.559 m2/g 的 SSABET 以及在较宽 pH 值范围(pH>3.08)内的阴离子表面,这些因素使 PPMB 成为水体系中高效、经济可行且环保的铈吸附剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


