Microscopic Scaling Relation of Ti-Based Catalysts in De/Hydrogenation Reactions of Mg/MgH2
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
While some early transition metals, such as Ti, can efficiently adsorb and dissociate hydrogen, they have rarely been utilized in hydrogenation and dehydrogenation (de/hydrogenation) reactions because their strong Cat–H bond results in a high hydrogen diffusion barrier. This limitation is known as the macroscopic scaling relation. Herein, using de/hydrogenation reactions of Mg/MgH2 as the example, we report that the hydrogen dissociation and diffusion barrier can be scaled by the Ti valence state, leading to the establishment of a “microscopic” scaling relation. The reaction rates of TiTM-MgO/MgH2 are improved by 69–72 times compared to that of MgH2 under the same conditions, which are even 10 times higher than those of Pd- and Pt-based catalysts. Kinetic analyses and density functional theory (DFT) calculations confirm that the electron transfer properties between catalysts and hydrogens can be systematically controlled as a function of Ti valence states, optimizing the Ti–H bond stability. Significantly, the chemical and structural properties of the TiTM-MgO catalyst remained largely unchanged during and after de/hydrogenation reactions. Our results revealed a “microscopic” scaling relation within a single element governed by its valence state, offering a blueprint for the application of early transition metals in de/hydrogenation reactions.
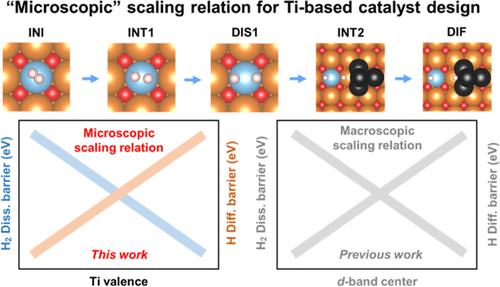
钛基催化剂在镁/镁氢化反应中的微观缩放关系
虽然一些早期的过渡金属(如钛)可以有效地吸附和离解氢,但它们很少被用于氢化和脱氢(脱氢/氢化)反应,因为它们的强 Cat-H 键导致氢扩散障碍很高。这种限制被称为宏观缩放关系。在此,我们以 Mg/MgH2 的脱氢/加氢反应为例,报告了氢的离解和扩散障碍可以通过 Ti 价态进行缩放,从而建立了 "微观 "缩放关系。在相同条件下,TiTM-MgO/MgH2 的反应速率比 MgH2 提高了 69-72 倍,甚至比钯基和铂基催化剂高出 10 倍。动力学分析和密度泛函理论(DFT)计算证实,催化剂与氢原子之间的电子转移特性可作为 Ti 价态的函数进行系统控制,从而优化了 Ti-H 键的稳定性。值得注意的是,TiTM-MgO 催化剂的化学和结构特性在脱氢/加氢反应期间和之后基本保持不变。我们的研究结果揭示了单一元素内部受其价态制约的 "微观 "比例关系,为早期过渡金属在脱氢/加氢反应中的应用提供了蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


