Utilizing Waste Biomass from Agricultural and Forestry Sustainable Resources: Biomass Conversion to Functional Adsorbent Material for Efficient Removal of Total Phosphate in Practical Water
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
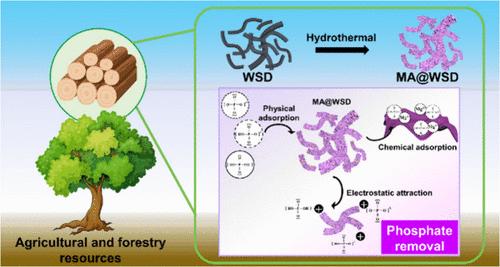
The promotion of the utilization of waste agricultural and forestry resources (AFRs) as a means of combating environmental pollution represents an advanced and necessary development approach with the potential to achieve sustainable development. In this work, an advanced adsorbent with a functional Mg–Al bimetallic layer was prepared using waste sawdust biomass (WSD) as a raw material. The layer serves as the primary active component for adsorbing total phosphate from both simulated and real wastewater. The hierarchical structure of the Mg–Al bimetallic hydroxide-modified waste sawdust biomass (MA@WSD) has an abundance of nanosheets on its surface, providing ample binding sites and an enhanced specific surface area of 175.99 m2/g. Under the optimal conditions, the maximum removal efficiency toward phosphate can reach 99.99%. The adsorption of phosphate by MA@WSD follows the pseudo-second order kinetic (PSOK) model, indicating that chemisorption is the rate-determining step. Moreover, the thermodynamic data demonstrate the spontaneous nature of the adsorption process, indicating the favorable characteristics of the developed material. The adsorption mechanism can be summarized as the collaboration of physical adsorption, electrostatic interaction, and chemical adsorption. The results of the regeneration process indicate that MA@WSD exhibits a retention of 50.3% of its initial adsorption performance following the fifth testing cycle, thereby suggesting its potential for comparable reusability. The MA@WSD performs well in adsorbing total phosphate in real river water, and the removal efficiency of MA@WSD is evidently superior to commercial activated carbon, which is at least 70% higher than that of the commercial activated carbon. Besides, the 5-time average removal efficiency toward total phosphate by MA@WSD is 62.9%, evidently higher than the 29.1% of commercially available activated carbon, indicating its potential as an alternative for treating phosphorus-containing wastewater. The research provides theoretical support for harmless treatment, resource utilization, carbon sequestration, and emission reduction of waste biomass.
利用来自农林业可持续资源的废弃生物质:生物质转化为功能性吸附材料以高效去除实用水中的总磷酸盐
推广利用废弃农林资源(AFR)作为治理环境污染的一种手段,是一种先进而必要的发展方式,具有实现可持续发展的潜力。本研究以废弃锯末生物质(WSD)为原料,制备了一种具有镁铝双金属功能层的先进吸附剂。该层是吸附模拟废水和实际废水中总磷酸盐的主要活性成分。氢氧化镁-铝双金属改性废锯末生物质(MA@WSD)的分层结构表面有大量纳米片,提供了大量的结合位点,比表面积提高到 175.99 m2/g。在最佳条件下,对磷酸盐的最大去除率可达 99.99%。MA@WSD 对磷酸盐的吸附遵循伪二阶动力学(PSOK)模型,表明化学吸附是决定吸附速率的步骤。此外,热力学数据证明了吸附过程的自发性,表明了所开发材料的良好特性。吸附机理可概括为物理吸附、静电作用和化学吸附的协同作用。再生过程的结果表明,MA@WSD 在第五个测试周期后的吸附性能保留了初始吸附性能的 50.3%,从而表明其具有可比重复使用的潜力。MA@WSD对实际河水中总磷酸盐的吸附性能良好,其去除率明显优于商业活性炭,比商业活性炭至少高出70%。此外,MA@WSD 对总磷酸盐的 5 次平均去除率为 62.9%,明显高于市售活性炭的 29.1%,表明其具有替代处理含磷废水的潜力。该研究为废弃生物质的无害化处理、资源化利用、固碳和减排提供了理论支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


