Insights into the Surface Electronic Structure and Catalytic Activity of InOx/Au(111) Inverse Catalysts for CO2 Hydrogenation to Methanol
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
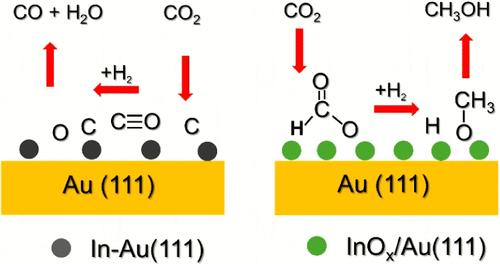
The direct conversion of carbon dioxide (CO2) into methanol (CH3OH) via low-temperature hydrogenation is crucial for recycling anthropogenic CO2 emissions and producing fuels or high value chemicals. Nevertheless, it continues to be a great challenge due to the trade-off between selectivity and catalytic activity. For CO2 hydrogenation, In2O3 catalysts are known for their high CH3OH selectivity. Subsequent studies explored depositing metals on In2O3 to enhance CO2 conversion. Despite extensive research on metal (M) supported In2O3 catalysts, the role of In–M alloys and M/In2O3 interfaces in CO2 activation and CH3OH selectivity remains unclear. In this work, we have examined the behavior of In/Au(111) alloys and InOx/Au(111) inverse systems during CO2 hydrogenation using synchrotron-based ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) and catalytic tests in a batch reactor. Indium forms alloys with Au(111) after deposition. The In–Au(111) alloys display high reactivity toward CO2 and can dissociate the molecule at room temperature to generate InOx nanostructures. At very low coverages of In (≤0.05 ML), the InOx nanostructures are not stable under CO2 hydrogenation conditions and the active In–Au(111) alloys produces mainly CO and little methanol. An increase in indium coverage to 0.3 ML led to stable InOx nanostructures under CO2 hydrogenation conditions. These InOx/Au(111) catalysts displayed a high selectivity (∼80%) toward CH3OH production and an activity for CO2 conversion that was at least 10 times larger than that of plain In2O3 or Cu(111) and Cu/ZnO(0001̅) benchmark catalysts. The results of AP-XPS show that InOx/Au(111) produces methanol via methoxy intermediates. Inverse oxide/metal catalysts containing InOx open up a possibility for improving CO2 → CH3OH conversion in processes associated with the control of environmental pollution and the production of high value chemicals.
深入了解 InOx/Au(111) 反相催化剂在二氧化碳加氢制甲醇过程中的表面电子结构和催化活性
通过低温加氢将二氧化碳(CO2)直接转化为甲醇(CH3OH),对于回收人为二氧化碳排放、生产燃料或高价值化学品至关重要。然而,由于选择性和催化活性之间的权衡,这仍然是一个巨大的挑战。对于 CO2 加氢,In2O3 催化剂以其高 CH3OH 选择性而著称。随后的研究探索了在 In2O3 上沉积金属以提高 CO2 转化率的方法。尽管对金属(M)支撑的 In2O3 催化剂进行了广泛的研究,但 In-M 合金和 M/In2O3 界面在 CO2 活化和 CH3OH 选择性中的作用仍不清楚。在这项工作中,我们利用同步辐射环境压力 X 射线光电子能谱(AP-XPS)和批量反应器中的催化测试,研究了 In/Au(111) 合金和 InOx/Au(111) 逆体系在 CO2 加氢过程中的行为。铟在沉积后与金(111)形成合金。In-Au(111) 合金对二氧化碳具有很高的反应活性,可在室温下解离二氧化碳分子,生成 InOx 纳米结构。在极低的铟覆盖率(≤0.05 ML)条件下,InOx 纳米结构在二氧化碳氢化条件下并不稳定,活性 In-Au(111)合金主要产生二氧化碳和少量甲醇。将铟的覆盖率提高到 0.3 ML 后,InOx 纳米结构在二氧化碳氢化条件下变得稳定。这些 InOx/Au(111) 催化剂对 CH3OH 的生产具有很高的选择性(∼80%),其 CO2 转化活性至少是普通 In2O3 或 Cu(111) 和 Cu/ZnO(0001̅) 基准催化剂的 10 倍。AP-XPS 的结果表明,InOx/Au(111) 通过甲氧基中间体产生甲醇。含有 InOx 的反相氧化物/金属催化剂为改善与控制环境污染和生产高价值化学品相关的工艺中 CO2 → CH3OH 的转化提供了可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


