An Aged Tree with a New Bloom: A Simple Spatiotemporal Programming Strategy Enables Carbon Dot Photosensitizers to Regulate Cell Pyroptosis for Enhanced Tumor Photodynamic-Immunotherapy
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Pyroptosis induced by photodynamic therapy (PDT) is a promising field in both PDT and immunotherapy for tumors. However, effectively inducing tumor cell pyroptosis while triggering a strong immune response using current photosensitizers remains challenging. Herein, the developed positively charged carbon dots (PCDs) nanoPSs were utilized to modulate tumor cell pyroptosis for the first time through a simple spatiotemporal programming strategy. Briefly, PCDs enabled precisely time-dependent targeting of the cell membrane or lysosome. Upon light irradiation, in vitro studies revealed that lysosome-targeted PDT primarily induced apoptosis, while membrane-targeted PDT triggered pyroptosis, resulting in enhanced PDT efficacy and robust activation of the immune response. Conclusively, in vivo studies demonstrated that PCDs could serve as a novel pyroptosis nanotuner for enhanced photodynamic-immunotherapy, thereby simultaneously eliminating primary tumors and inhibiting distant tumor growth and metastases. This spatiotemporal programming strategy unprecedentedly offers a rejuvenation of aged PSs and expands the biomedical use of CDs in immunotherapy.
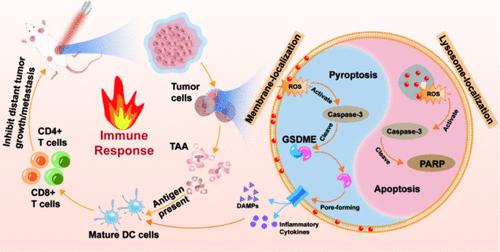
老树开新花:一种简单的时空编程策略可使碳点光敏剂调节细胞跃迁,从而增强肿瘤光动力免疫疗法的效果
光动力疗法(PDT)诱导的热休克是光动力疗法和肿瘤免疫疗法中一个前景广阔的领域。然而,使用目前的光敏剂在有效诱导肿瘤细胞发生热休克的同时引发强烈的免疫反应仍具有挑战性。在这里,我们首次利用开发的带正电荷的碳点(PCDs)纳米PSs,通过简单的时空编程策略来调节肿瘤细胞的热休克。简而言之,PCDs 能够精确地根据时间定向细胞膜或溶酶体。体外研究发现,在光照射下,以溶酶体为靶点的光致透射疗法主要诱导细胞凋亡,而以细胞膜为靶点的光致透射疗法则引发细胞猝死,从而提高了光致透射疗法的疗效,并有力地激活了免疫反应。最终,体内研究表明,PCDs 可以作为一种新型的热凋亡纳米调谐器,用于增强光动力免疫疗法,从而同时消除原发肿瘤并抑制远处肿瘤的生长和转移。这种时空编程策略史无前例地使老化的 PSs 重获新生,并拓展了 CDs 在免疫疗法中的生物医学应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


