Poly(bis(1-methylpiperazin-1-ium-amide) Nanofilm Composite Membrane with Nanochannel‑Enabled Microporous Structure and Enhanced Steric Hindrance for Magnesium/Lithium Separation
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Efficient lithium/magnesium (Li+/Mg2+) separation attainment is fundamental to the extraction of lithium from brine by nanofiltration membrane separation process, which is essential for resource recovery and a circular water economy. However, for poly(piperazine-amide) nanofilm composite membranes, the higher electronegativity affects the Mg2+ rejection and consequently Li+/Mg2+ separation performance. Manipulating the positive charge density and pore size regulation of the nanofiltration membranes are determinative of the Li+/Mg2+ separation performance improvement. Here, a new monomer 1,1′-(hexane-1,6-diyl)bis(1-methylpiperazin-1-ium) bromide containing bis-quaternary ammonium cations is employed as a molecular building block to fabricate polyamide nanofilms via interfacial polymerization. The dual quaternary ammoniums and the rod-shaped conformation of the monomer confer enhanced electropositivity, steric hindrance, loosely packed microporous network structure (pore diameter∼0.8–1.35 nm), and high free volume. The resultant membrane exhibits high water permeance of 28.34 L m−2 h−1 bar−1 with good Li+/Mg2+ selectivity of up to 76.9. In addition, the membrane also exhibits chlorine stability performance owing to the lack of the chlorine sensitive −NH groups in the formed tertiary amide structures. Computational insights on the structural properties, nanofilm formation, and transmembrane water and ion transport behaviors are provided. This study offers insightful theoretical and technological concepts to design and construct membrane materials for energy-efficient separations.
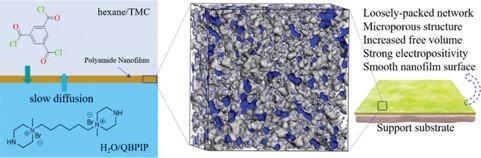
具有纳米通道微孔结构和增强立体阻碍作用的双(1-甲基哌嗪-1-鎓-酰胺)聚纳米薄膜复合膜用于镁/锂分离
高效的锂/镁(Li+/Mg2+)分离是利用纳滤膜分离工艺从盐水中提取锂的基础,这对于资源回收和循环水经济至关重要。然而,对于聚(哌嗪-酰胺)纳米膜复合膜而言,较高的电负性会影响 Mg2+ 的排斥,进而影响 Li+/Mg2+ 的分离性能。调节纳滤膜的正电荷密度和孔径大小是提高 Li+/Mg2+ 分离性能的决定性因素。在这里,一种含有双季铵阳离子的新型单体 1,1′-(己烷-1,6-二基)双(1-甲基哌嗪-1-鎓)溴化物被用作分子构件,通过界面聚合制造聚酰胺纳米膜。单体的双季铵和杆状构象增强了电正性、立体阻碍、松散的微孔网络结构(孔径∼0.8-1.35 nm)和高自由体积。结果膜的透水率高达 28.34 L m-2 h-1 bar-1,对 Li+/Mg2+ 的选择性高达 76.9。此外,由于形成的三级酰胺结构中缺乏对氯敏感的 -NH 基团,该膜还具有氯稳定性能。本研究提供了有关结构特性、纳米膜形成、跨膜水和离子传输行为的计算见解。这项研究为设计和构建高能效分离膜材料提供了深刻的理论和技术概念。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


