Chitosan-melanin complex microsphere: A potential colonic delivery system for protein drugs
IF 10.7
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The characteristics and performance of chitosan-based colon delivery systems are significantly influenced by the method of preparation. Insect chitosan-melanin complex (CMC) may offer superior attributes over traditional shrimp and crab chitosan (CS) for colon-targeted administration. This study used dung beetle CMC as the carrier matrix and comprehensively examined the impact of various crosslinking techniques on the colonic drug delivery efficacy of microspheres, encompassing drug loading, swelling, drug release behavior, adhesion, enzymatic degradation, and absorption enhancement. The results indicate that F-TPPLC microspheres, crosslinked with a combination of formaldehyde and sodium tripolyphosphate, exhibit superior drug loading capabilities, optimal swelling behavior, and controlled in vitro drug release profiles in the colonic environment, along with excellent adhesion and enzymatic degradation properties within intestinal tract. Notably, these F-TPPLC microspheres increase paracellular permeability, possibly by disrupting the calcium-dependent adhesion junctions. In comparison to commercial CS, CMC demonstrates superior drug encapsulation efficiency, enhanced colonic drug release, adhesion, and absorption promotion, rendering it a favorable candidate as a carrier in colon-targeted drug delivery systems. Consequently, F-TPPLC microspheres derived from CMC are highly suitable for colon drug delivery applications and show promising potential for the oral delivery of peptide and protein-based therapeutics to the colon.
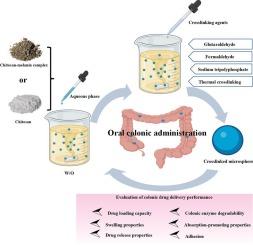
壳聚糖-黑色素复合微球:一种潜在的蛋白质药物结肠输送系统
壳聚糖结肠给药系统的特性和性能受到制备方法的很大影响。在结肠靶向给药方面,昆虫壳聚糖-黑色素复合物(CMC)可能比传统的虾蟹壳聚糖(CS)具有更优越的特性。本研究以蜣螂壳聚糖-麦拉宁复合物(CMC)为载体基质,全面考察了各种交联技术对微球结肠给药效果的影响,包括药物负载、溶胀、药物释放行为、粘附、酶降解和吸收增强等方面。结果表明,用甲醛和三聚磷酸钠组合交联的 F-TPPLC 微球在结肠环境中表现出卓越的载药能力、最佳的溶胀行为和可控的体外药物释放曲线,同时在肠道内具有出色的粘附性和酶降解特性。值得注意的是,这些 F-TPPLC 微球可能通过破坏钙依赖性粘附连接,增加了细胞旁通透性。与商用 CS 相比,CMC 表现出更高的药物封装效率,增强了结肠药物释放、粘附和吸收促进作用,使其成为结肠靶向给药系统载体的有利候选材料。因此,由 CMC 制成的 F-TPPLC 微球非常适合结肠给药应用,并显示出向结肠口服多肽和蛋白质类治疗药物的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


