F-modified Cu/electrolyte interface boosts CO2 electroreduction into C2H4 and C2H5OH products via an alternative CC coupling mechanism
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
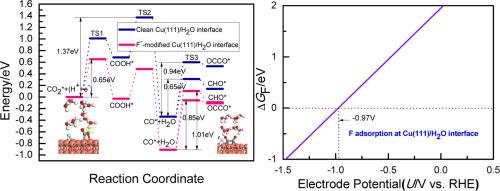
A F−-modified Cu(111) electrocatalysts exhibited an ultrahigh Faradaic efficiency and product selectivity towards CO2 electroreduction into C2 products. Thus, CO2 electroreduction mechanisms towards C2H4 and C2H5OH products at F−-modified Cu(111)/H2O interface based on DFT calculations are studied in this work, by which the influencing mechanism of specifically adsorbed F− on electroreduction activity, product selectivity and a alternative C![]() C coupling mechanism can be revealed. Our present studies indicate that the presence of F− can notably enhance CO2 electroreduction activity towards key intermediate CO because of significantly changed CO2 adsorption configuration and weakening effect of F− on COOH adsorption strength. CO dimerization into dimer OCCO through Langmuir-Hinshelwood mechanism is more favorable than CHO formation in the presence of the F−. Thus, it can be concluded that C
C coupling mechanism can be revealed. Our present studies indicate that the presence of F− can notably enhance CO2 electroreduction activity towards key intermediate CO because of significantly changed CO2 adsorption configuration and weakening effect of F− on COOH adsorption strength. CO dimerization into dimer OCCO through Langmuir-Hinshelwood mechanism is more favorable than CHO formation in the presence of the F−. Thus, it can be concluded that C![]() C coupling reaction occurs via CO dimerization. More CO adsorption strength because of modification of F− can explain easier occurrence of C
C coupling reaction occurs via CO dimerization. More CO adsorption strength because of modification of F− can explain easier occurrence of C![]() C coupling reaction, thus leading to change of product selectivity. The parallel OCCOH and OCCHO pathways are proposed for CO2 electroreduction into C2H4 and C2H5OH products at F−-modified Cu(111)/H2O interface, in which CO dimerization is regarded as the rate-determining step. Ou present studies can unveil CO2 electroreduction mechanisms and significant role of the specifically adsorbed F− at Cu/electrolyte interface for promoting CO2 electroreduction activity and improving product selectivity towards C2 products.
C coupling reaction, thus leading to change of product selectivity. The parallel OCCOH and OCCHO pathways are proposed for CO2 electroreduction into C2H4 and C2H5OH products at F−-modified Cu(111)/H2O interface, in which CO dimerization is regarded as the rate-determining step. Ou present studies can unveil CO2 electroreduction mechanisms and significant role of the specifically adsorbed F− at Cu/electrolyte interface for promoting CO2 electroreduction activity and improving product selectivity towards C2 products.
F 改性铜/电解质界面通过另一种 CC 耦合机制促进 CO2 电还原成 C2H4 和 C2H5OH 产物
一种 F 修饰的 Cu(111) 电催化剂在将 CO2 电还原成 C2 产物方面表现出了超高的法拉第效率和产物选择性。因此,本研究基于 DFT 计算,研究了 F- 改性 Cu(111)/H2O 界面上 CO2 电还原 C2H4 和 C2H5OH 产物的机理,从而揭示了特异性吸附 F- 对电还原活性、产物选择性的影响机理以及另一种 CC 耦合机理。本研究表明,由于 F- 显著改变了 CO2 的吸附构型,并削弱了 F- 对 COOH 的吸附强度,因此 F- 的存在能显著提高 CO2 对关键中间产物 CO 的电还原活性。在 F- 存在的情况下,CO 通过 Langmuir-Hinshelwood 机理二聚为二聚体 OCCO 比 CHO 的形成更有利。因此,可以得出结论,CC 偶联反应是通过 CO 二聚作用发生的。由于 F- 的改性,CO 的吸附力更大,这可以解释为什么 CC 偶联反应更容易发生,从而导致产物选择性的改变。在 F- 修饰的 Cu(111)/H2O 界面上,CO2 电还原成 C2H4 和 C2H5OH 产物的平行 OCCOH 和 OCCHO 途径被提出,其中 CO 二聚化被认为是决定速率的步骤。本研究揭示了 CO2 电还原机制,以及 Cu/电解质界面上特异吸附的 F- 在促进 CO2 电还原活性和提高 C2 产物选择性方面的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


