Enhancement of ionic conductivity and air stability by co-doping Li10SnP2S12 with Nb and O
IF 8.9
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
Li10SnP2S12 represents a quintessential sulfide solid-state electrolyte that has garnered considerable interest within the domain of solid-state batteries. However, it still has much room for enhancing its ionic conductivity and air stability. In this paper, Li10SnP2S12 was modified by solid-phase sintering method using different doping amounts of Nb2O5. XRD refinement confirmed that Nb and O elements were successfully doped into the P and S sites, in which the ionic conductivity of Li10SnP1.96Nb0.04S11.9O0.1 was up to 2.93 mS cm−1 at room temperature. After Nb2O5 doping, the lattice volume expands due to the larger ionic radius of Nb5+ compared to P5+. This expansion broadens the channels through which lithium ions travel, thereby enhancing Li+ conductivity. O doping improves the stability of the sulfide against moisture and forms a strong P![]() O bond with P, which resists further oxidation. The air stability test demonstrated enhanced air stability of the doped electrolyte. The AC impedance test conducted at various temperatures confirmed that the doped electrolyte exhibits lower activation energy, which facilitates an enhancement in Li+ conductivity. Finally, the assembled Li-In/Li10SnP1.96Nb0.04S11.9O0.1/LNO@LCO battery exhibits higher first turn discharge specific capacity and good cycle stability.
O bond with P, which resists further oxidation. The air stability test demonstrated enhanced air stability of the doped electrolyte. The AC impedance test conducted at various temperatures confirmed that the doped electrolyte exhibits lower activation energy, which facilitates an enhancement in Li+ conductivity. Finally, the assembled Li-In/Li10SnP1.96Nb0.04S11.9O0.1/LNO@LCO battery exhibits higher first turn discharge specific capacity and good cycle stability.
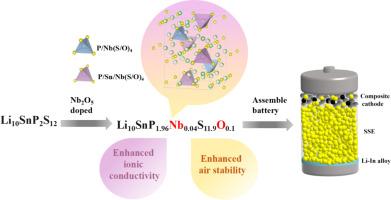
通过在 Li10SnP2S12 中掺杂 Nb 和 O 增强离子导电性和空气稳定性
Li10SnP2S12 是一种典型的硫化物固态电解质,在固态电池领域备受关注。然而,它的离子导电性和空气稳定性仍有很大的提升空间。本文采用固相烧结法,使用不同掺杂量的 Nb2O5 对 Li10SnP2S12 进行了改性。XRD 精炼证实,Nb 和 O 元素成功地掺杂到了 P 位和 S 位,其中 Li10SnP1.96Nb0.04S11.9O0.1 在室温下的离子电导率高达 2.93 mS cm-1。掺杂 Nb2O5 后,由于 Nb5+ 的离子半径比 P5+ 大,晶格体积膨胀。这种膨胀拓宽了锂离子通过的通道,从而增强了 Li+ 的导电性。掺杂 O 可提高硫化物的防潮稳定性,并与 P 形成牢固的 PO 键,从而抵抗进一步氧化。空气稳定性测试表明,掺杂电解质的空气稳定性得到了增强。在不同温度下进行的交流阻抗测试证实,掺杂电解质的活化能较低,这有利于提高 Li+ 的导电性。最后,组装好的 Li-In/Li10SnP1.96Nb0.04S11.9O0.1/LNO@LCO 电池显示出更高的首次放电比容量和良好的循环稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of energy storage
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
11.80
自引率
24.50%
发文量
2262
审稿时长
69 days
期刊介绍:
Journal of energy storage focusses on all aspects of energy storage, in particular systems integration, electric grid integration, modelling and analysis, novel energy storage technologies, sizing and management strategies, business models for operation of storage systems and energy storage developments worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


