Cobalt(II) catalyzed Michael-type hydroamination of activated olefins
IF 1.8
3区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The aza-Michael addition reaction of primary and secondary amines to α,β-unsaturated olefins viz; acrylonitrile, phenyl vinyl sulfone and dimethyl maleate has been carried out using 5–10 mol% Co(NO3)2.6H2O as a catalyst in t-BuOMe at 80–100 °C, giving rise to the desired β-aminocarbonyl compounds or sulfones in moderate to good yields. A wide range of aromatic amines, even those bearing electron withdrawing groups could be added to activated olefins via this strategy. Addition of (hetero)aromatic amines were also feasible, while in case of 2-aminopyridine the reaction was found to be effective only when AgOTf was added along with the catalyst. The aliphatic amines; benzylamine, dibenzylamine, di-n-butylamine were also smoothly added to acrylonitrile and phenyl vinyl sulfone. The methodology describes cobalt(II) nitrate as an eco-friendly, cheap and shelf available catalyst suitable for performing the Michael-type hydroamination reactions.
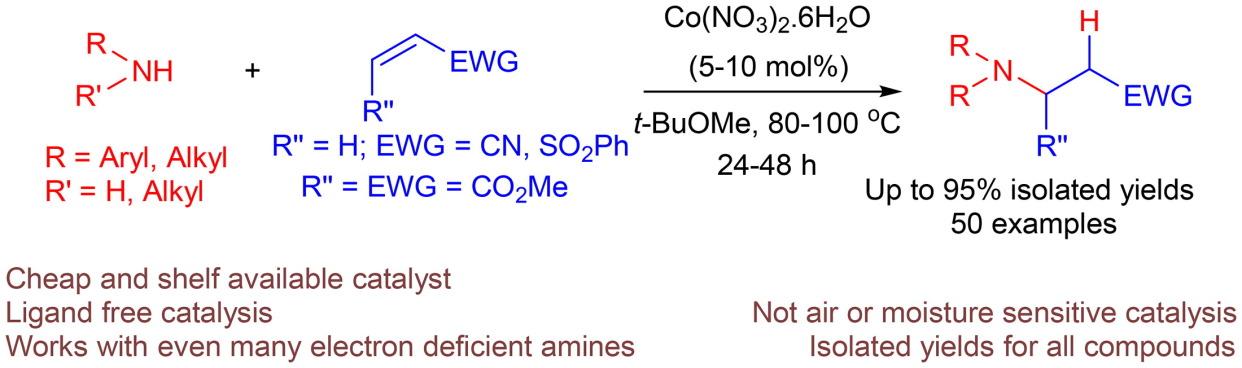
钴(II)催化活化烯烃的迈克尔型加氢反应
以 5-10 mol% 的 Co(NO3)2.6H2O 为催化剂,在 80-100 °C 的 t-BuOMe 溶液中进行了伯胺和仲胺与α,β-不饱和烯烃(即丙烯腈、苯基乙烯基砜和马来酸二甲酯)的氮杂迈克尔加成反应,得到了所需的β-氨基羰基化合物或砜,收率中等至良好。通过这种方法可以将多种芳香胺,甚至是那些带有取电子基团的芳香胺添加到活化烯烃中。添加(杂)芳香胺也是可行的,但对于 2-氨基吡啶,只有在添加 AgOTf 和催化剂时反应才有效。脂肪族胺;苄胺、二苄胺、二正丁胺也能顺利地加入到丙烯腈和苯乙烯砜中。该方法说明硝酸钴(II)是一种环保、廉价且可在货架上购买到的催化剂,适用于进行迈克尔型氢化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Synthetic Communications
化学-有机化学
CiteScore
4.40
自引率
4.80%
发文量
156
审稿时长
4.3 months
期刊介绍:
Synthetic Communications presents communications describing new methods, reagents, and other synthetic work pertaining to organic chemistry with sufficient experimental detail to permit reported reactions to be repeated by a chemist reasonably skilled in the art. In addition, the Journal features short, focused review articles discussing topics within its remit of synthetic organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


