Novel waste wool fabric reinforced alginate-gum hydrogel composites for rapid and selective Pb (II) adsorption
IF 6.2
Q1 CHEMISTRY, APPLIED
Carbohydrate Polymer Technologies and Applications
Pub Date : 2024-11-04
DOI:10.1016/j.carpta.2024.100601
引用次数: 0
Abstract
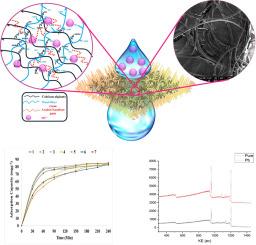
Heavy metals employed in various industrial applications can negatively impact both the ecosystem and human beings. Common techniques for eliminating pollutants often rely on expensive materials. So, this study focuses on exploring economical alternatives obtained from nature and textile waste. In this study, a hydrogel composite was synthesized using wool nonwoven fabric mixed with alginate, gum Arabic (GA), and xanthan gum (XG) to evaluate its efficacy in adsorbing lead (Pb) from aqueous solutions. The composites were characterized using SEM, FTIR, and XPS to understand their structure and composition before and after Pb adsorption. The effects of time, pH, and initial metal ion concentration on Pb adsorption by the composite were also investigated. Maximum adsorption was observed at a basic pH, with the highest value recorded at 85.2 mg/g. Notably, 88.2 % of this maximum adsorption was achieved within 60 min, indicating a rapid adsorption process. Kinetic studies indicated that the adsorption process best fits pseudo-second-order kinetics, while the Freundlich model, with an R² value of 0.95, suggests a chemisorption mechanism. The developed wool-alginate-gum hydrogel composite has shown to be a promising candidate for the removal of Pb²⁺ ions from wastewater.
用于快速、选择性吸附铅 (II) 的新型废羊毛织物增强海藻酸胶水凝胶复合材料
各种工业应用中使用的重金属会对生态系统和人类造成负面影响。消除污染物的常用技术往往依赖于昂贵的材料。因此,本研究侧重于探索从自然界和纺织废料中获取的经济替代品。本研究使用羊毛无纺布与海藻酸盐、阿拉伯树胶(GA)和黄原胶(XG)混合合成了一种水凝胶复合材料,以评估其吸附水溶液中铅(Pb)的功效。使用扫描电镜、傅立叶变换红外光谱和 XPS 对复合材料进行了表征,以了解其吸附铅前后的结构和组成。此外,还研究了时间、pH 值和初始金属离子浓度对复合材料吸附铅的影响。在碱性 pH 值下,吸附量最大,最高值为 85.2 mg/g。值得注意的是,最大吸附量的 88.2% 是在 60 分钟内实现的,这表明吸附过程非常迅速。动力学研究表明,吸附过程最符合伪二阶动力学,而 Freundlich 模型的 R² 值为 0.95,表明了化学吸附机制。研究表明,所开发的羊毛-海藻酸胶水凝胶复合材料有望用于去除废水中的铅⁺离子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


