Phase evolution and elemental distribution of zinc and germanium during the sulfide roasting, zinc fuming and leaching processes: Benefit of pretreating zinc oxide dust
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
Germanium plays an essential role in many high-tech fields because of its excellent electrical and optical properties. As a zinc refining by-product, zinc oxide dust (ZOD) is one of the most important sources of germanium recovery. However, the low leaching efficiency of germanium seriously hinders germanium recovery. This study focuses on the phase evolution and elemental distribution of zinc and germanium during the zinc refining process by analyzing the occurrence state of zinc and germanium in products and the key factors and chemical reactions causing the loss of zinc and germanium during the process. In the sulfuric-acid leaching process without an oxidant, the sulfides encapsulating germanium cannot be leached, which resulted in a loss of germanium. In addition, the formation of an insoluble PbSO4 coating layer and silica compounds during the sulfuric-acid leaching impeded the leaching reactions. Accordingly, pre-treatment by oxidative leaching with Fe3+ and the role of NaAc in dissolving PbSO4 were tested. The efficiency of Ge recovery is approximately 30 % higher than that of the conventional leaching process.
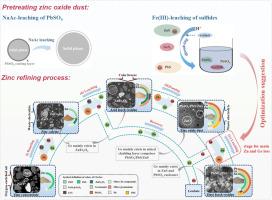
硫化物焙烧、锌发烟和浸出过程中锌和锗的相变和元素分布:氧化锌粉尘预处理的益处
锗因其优异的电气和光学特性,在许多高科技领域发挥着重要作用。作为锌精炼的副产品,氧化锌粉尘(ZOD)是锗回收的重要来源之一。然而,锗的低浸出效率严重阻碍了锗的回收。本研究通过分析锌和锗在产品中的出现状态,以及在锌精炼过程中造成锌和锗损失的关键因素和化学反应,重点研究了锌精炼过程中锌和锗的相演变和元素分布。在没有氧化剂的硫酸浸出过程中,包裹锗的硫化物无法被浸出,从而导致锗的损失。此外,硫酸浸出过程中形成的不溶性 PbSO4 涂层和二氧化硅化合物也阻碍了浸出反应。因此,测试了用 Fe3+ 进行氧化浸出的预处理以及 NaAc 在溶解 PbSO4 中的作用。Ge 的回收效率比传统浸出工艺高出约 30%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


