Evaluation of thiocarbohydrazide derivative as corrosion inhibitor for C1018 carbon steel in 3.5% NaCl + 500ppm HAc solution: Electrochemical, SEM, FTIR and computational studies
IF 2.7
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
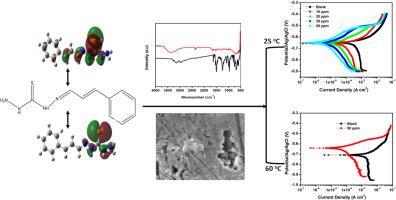
N'-((E)-3-phenylallylidene)hydrazinethiocarbohydrazide (PHCT) was synthesized, purified, characterized and assessed for its inhibitive property against the sweet corrosion of C1018 carbon steel in CO2-saturated 3.5 % NaCl + 500 ppm HAc solution at 25 and 60 °C. At 50 ppm and 25 °C, electrochemical impedance spectroscopy (EIS) confirmed that the presence of PHCT in the electric double layer fortified the hydrophobicity of the region and mitigated rate of charge transfer. Potentiodynamic polarization (PP) measurements at 25 °C showed that PHCT is a mixed-type inhibitor, which could reduce corrosion rate from 24.32 mpy in blank to 0.67 mpy at 50 ppm dosage and deliver 97.24 % inhibition efficiency. PHCT adsorbs, principally, using its NH2, C = S and C = C functional groups, based on FTIR characterization. Computational modeling using the FMO and MEP analyses confirmed that PHCT exhibits higher electron donating than electron accepting power and interacts with the metal surface through nitrogen and sulfur atoms. Scanning electron microscopy (SEM) confirmed the ability of PHCT to protect the steel surface from localized pitting corrosion. Although increasing the temperature up to 60 °C diminished the efficiency of PHCT, the inhibitor was able to provide up to 92 % efficiency.
评估硫代酰肼衍生物作为 C1018 碳钢在 3.5% NaCl + 500ppm HAc 溶液中的缓蚀剂:电化学、扫描电镜、傅立叶变换红外光谱和计算研究
对 N'-((E)-3-苯基亚烯丙基)肼基硫代甲酰肼(PHCT)进行了合成、纯化和表征,并评估了其在二氧化碳饱和的 3.5 % NaCl + 500 ppm HAc 溶液中于 25 和 60 °C 下对 C1018 碳钢甜腐蚀的抑制性。在 50 ppm 和 25 °C条件下,电化学阻抗谱(EIS)证实,双电层中 PHCT 的存在增强了该区域的疏水性,降低了电荷转移速率。25 °C 时的电位极化(PP)测量结果表明,PHCT 是一种混合型抑制剂,在 50 ppm 的剂量下可将腐蚀速率从空白时的 24.32 mpy 降低到 0.67 mpy,抑制效率为 97.24%。根据傅立叶变换红外光谱分析,PHCT 主要利用其 NH2、C = S 和 C = C 官能团进行吸附。利用 FMO 和 MEP 分析建立的计算模型证实,PHCT 的电子供能高于电子受能,并通过氮原子和硫原子与金属表面相互作用。扫描电子显微镜(SEM)证实了 PHCT 保护钢表面免受局部点腐蚀的能力。虽然温度升高到 60 ℃ 会降低 PHCT 的效率,但这种抑制剂的效率仍高达 92%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Scientific African
Multidisciplinary-Multidisciplinary
CiteScore
5.60
自引率
3.40%
发文量
332
审稿时长
10 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


