Synthesis of hierarchical porous silica aerogel for CO2 adsorption using decarbonized coal gasification fine slag
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
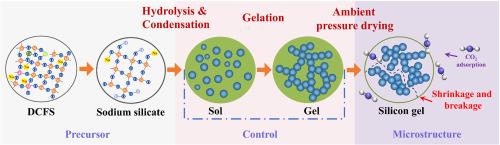
The preparation of hierarchical porous silica aerogels from coal gasification fine slag (CGFS) offers an effective approach to achieving high-value utilization of solid waste and reducing the production cost of solid adsorbent matrix materials. However, the main challenges involve overcoming technical barriers to efficiently and value-added conversion of CGFS into silica aerogels with CO₂ adsorption properties, as well as elucidating the phase transformation mechanisms during the synthesis process. In this study, CGFS was used as the raw material to obtain a silicon-containing precursor through pre-decarbonization (with ash content as high as 99.82 %) and alkali dissolution treatment. A hierarchical porous silica aerogel was then synthesized using an efficient hydrothermal process. The effect of alkali dissolution on silicon extraction and the phase transformation mechanisms were thoroughly discussed, and the leaching mechanism was analyzed through thermodynamic and kinetic models. The results showed that the high leaching rate of silicon was attributed to the presence of a large amount of amorphous SiO₂ in the decarbonized fine slag (DCFS), while the formation of zeolite Na-P1 and hydroxysodalite during the alkali dissolution process affected the efficiency of silicon extraction. Then, the structural formation mechanism and CO₂ adsorption properties of the hierarchical porous silica aerogels were analyzed using N₂ adsorption-desorption and CO₂-TPD. The SiO₂-1-30-0.5 exhibited a high CO₂ adsorption ability of 1.53 mmol g−1, and the CO₂ adsorption capacity maintained 94.78 % of the original value and after 5 adsorption-desorption cycles.
利用脱碳煤气化细渣合成用于二氧化碳吸附的分层多孔二氧化硅气凝胶
利用煤气化细渣(CGFS)制备分层多孔二氧化硅气凝胶是实现固体废弃物高值化利用和降低固体吸附基质材料生产成本的有效方法。然而,将煤气化细渣高效、增值地转化为具有 CO₂ 吸附性能的二氧化硅气凝胶所面临的主要挑战包括克服技术障碍,以及阐明合成过程中的相变机理。本研究以 CGFS 为原料,通过预脱碳(灰分含量高达 99.82%)和碱溶解处理获得含硅前驱体。然后采用高效水热法合成了分层多孔二氧化硅气凝胶。深入讨论了碱溶解对硅萃取的影响和相变机理,并通过热力学和动力学模型分析了浸出机理。结果表明,硅的高浸出率是由于脱碳细渣(DCFS)中存在大量无定形 SiO₂,而碱溶解过程中沸石 Na-P1 和羟基钠长石的形成影响了硅的萃取效率。然后,利用N₂吸附-解吸和CO₂-TPD分析了分层多孔二氧化硅气凝胶的结构形成机理和CO₂吸附特性。结果表明,SiO₂-1-30-0.5 对 CO₂ 的吸附能力高达 1.53 mmol g-1,在吸附-解吸循环 5 次后,CO₂ 的吸附容量仍保持在原始值的 94.78%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


